10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
23Introduction
1.8 US Clinical Study: Destination Therapy (continued)
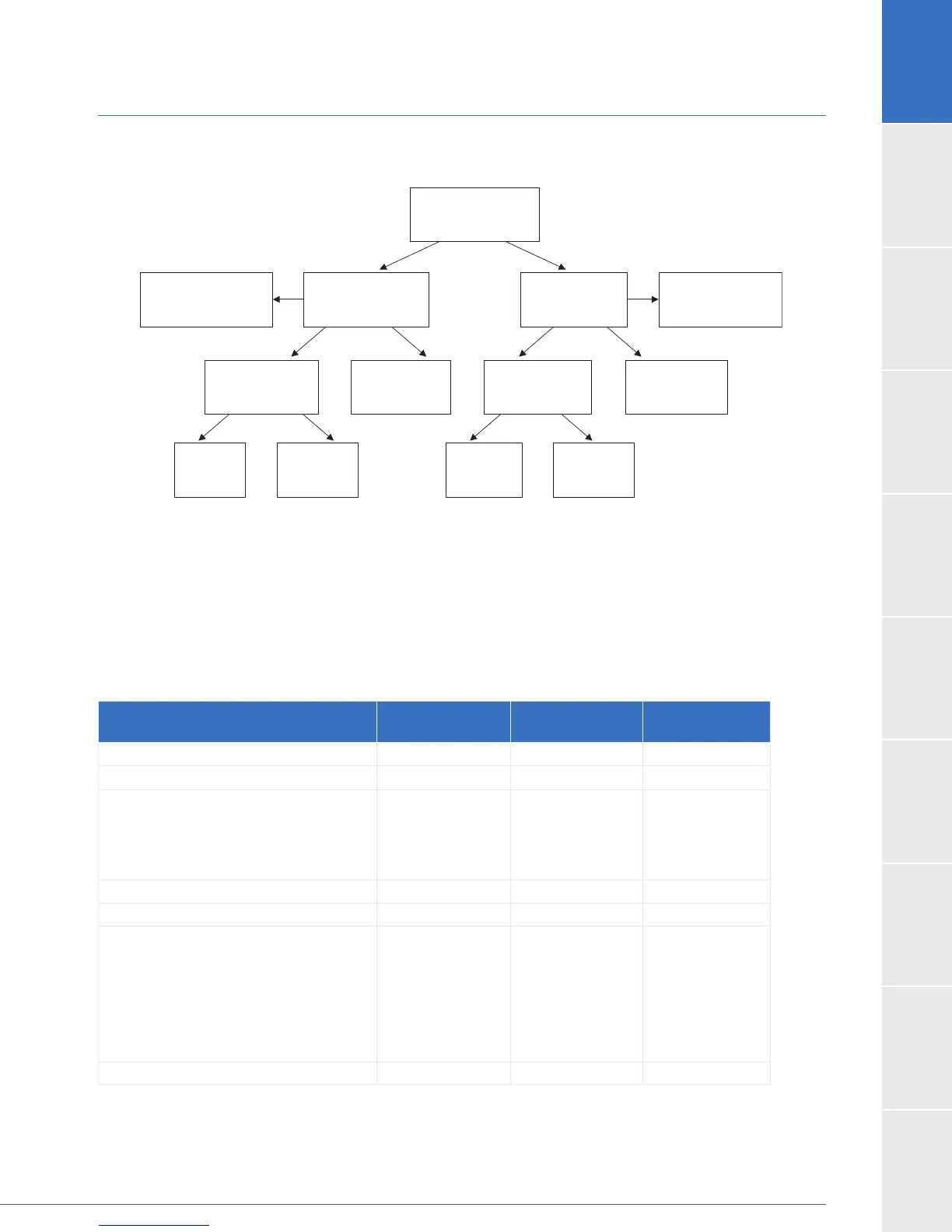
Figure 4:
Randomized Subjects
N = 300
Control
N = 100
Received Control
Device
N = 103
Received HeartWare
Device
N = 197
HeartWare N = 200
(96 Non-Sintered,
104 Sintered)
Did not complete
first 2 Years
N = 85
Completed first
2 Years
N = 115
Did not complete
first 2 Years
N = 32
Completed first
2 Years
N = 68
Death
N = 83
Voluntary
Withdrawal
N = 2
Death
N = 32
Voluntary
Withdrawal
N = 0
C. Study Population Demographics and Baseline Parameters
The demographics and baseline characteristics, as summarized in Table 11, are typical for an
Table 11: Patient Demographics and Baseline Characteristics in the rst 300 Subjects in the
ENDURANCE Trial
Demographics and Baseline
Characteristics
HVAD
Control
P-value
Age (years) 64.4 ± 12.0 66.1 ± 10.4 0.25
Male gender (%) 77.5% 80.8% 0.66
Race (%)
White
Black or African American
Other
79.5%
19.5%
1.0%
75.0%
25.0%
0.0%
0.37
Height (cm) 173.5 ± 9.8 175.2 ± 9.3 0.15
Body Surface Area (m
2
) 2.0 ± 0.3 2.0 ± 0.3 0.98
1
2
3
4
5-7
3.5%
27.5%
39.5%
21.5%
8.0%
1.0%
38.0%
41.0%
13.0%
7.0%
0.17
Ischemic Etiology of Heart Failure 59.5% 59.0%
 Loading...
Loading...