24 HVAD® Instructions for Use
10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
Pump Overview
Introduction
1.8 US Clinical Study: Destination Therapy (continued)
D. Safety and Effectiveness Results
1. Primary Endpoint
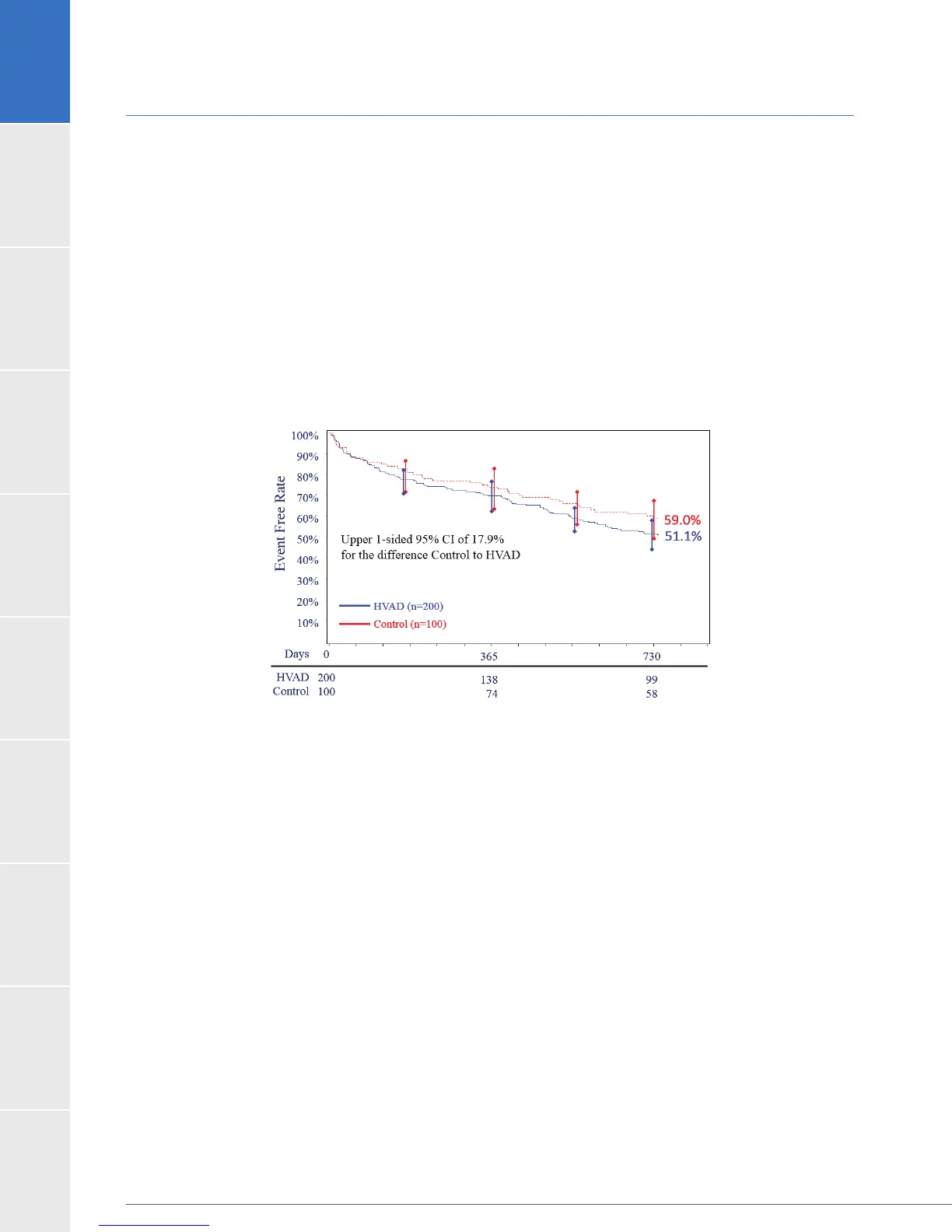
years post implantation. The Kaplan-Meier estimate for stroke-free success at 2 years for the
Control arm was 59.0%; as such, the interim analysis represented the primary analysis for the
arm was 51.1%. The results of the interim analysis are shown in Figure 5. The upper bound
(17.9%), resulting in a p-value of 0.1219. The interim analysis showed that the trial failed to
demonstrate non-inferiority of the HVAD to the Control.
Figure 5:4) and
alive on the originally implanted device, or transplanted or explanted for recovery.
Table 12.
 Loading...
Loading...