10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
25Introduction
1.8 US Clinical Study: Destination Therapy (continued)
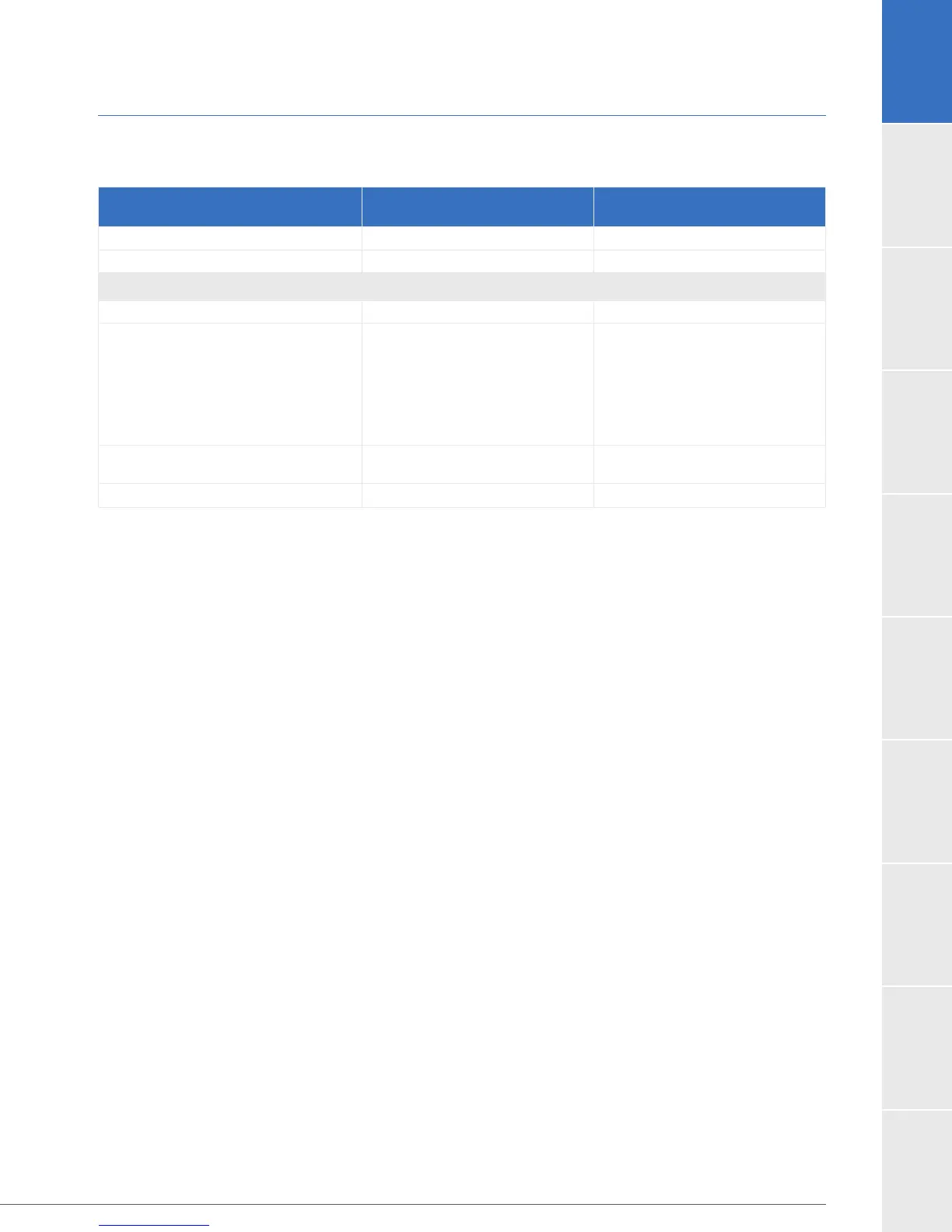
Table 12: Binary Analysis of the Primary ENDURANCE Endpoint and its Components for Subjects
Receiving Study or Control Device
Event Free Survival at 2 years
HVAD
Control
Success 51.5% (103) 59 (59.0%)
Failure 48.5% (97) 41.0% (41)
If Failure, reason:
Patient dies 35.5% (71) 25.0% (25)
Device malfunction or failure
requiring exchange, explant or
urgent transplant
Exchange
Explant
Urgent Transplant
11.0% (22)
9.5% (19)
0.0% (0)
1.5% (3)
16.0% (16)
14.0% (14)
0.0% (0)
2.0% (2)
Disabling stroke
1.5% (3) 0.0% (0)
Imputed failure* 0.5% (1) 0.0% (0)
*Patient experienced a stroke prior to their 2-year endpoint, and died beyond the 2 year endpoint, but
before the 24 week MRS assessment.
2. Secondary Endpoints
hypotheses associated with the secondary endpoints of incidence of bleeding (per
survival (time to death) could not be tested. As such, the secondary endpoints were not
reported.
3. Other Results - Adjunctive analysis: Primary Endpoint Using Expanded Dataset
Following the interim analysis at 300 patients, the trial was expanded to enroll additional
patients to further investigate various device, procedural, and clinical changes introduced
during the trial. A total of 451 patients (inclusive of the initial 300 patients) were enrolled, of
which 445 were implanted with a device. The patient disposition is summarized in Figure 6.
The results of the expanded dataset are summarized below.
 Loading...
Loading...