10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
27Introduction
1.8 US Clinical Study: Destination Therapy (continued)
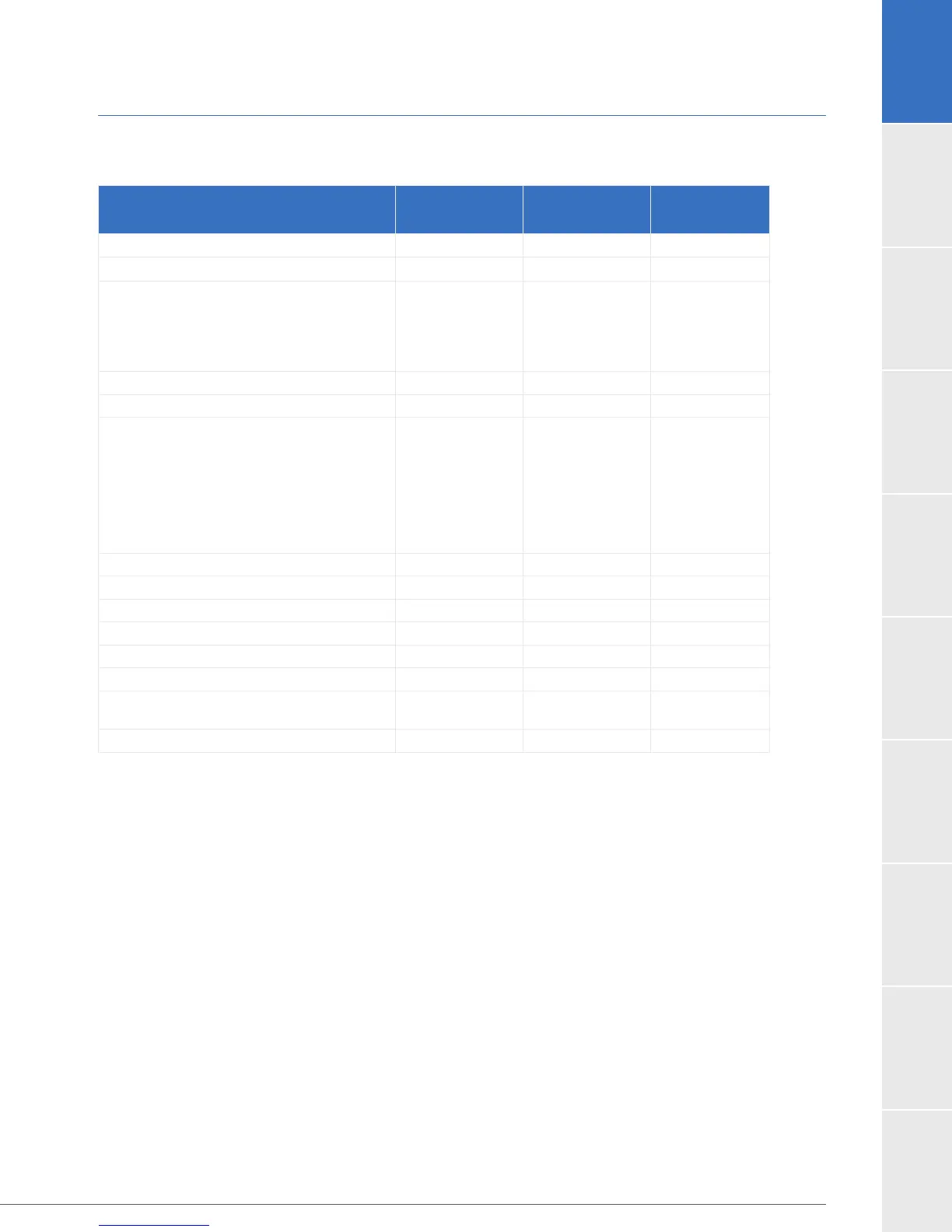
Table 13: Patient Demographics and Baseline Characteristics of the ENDURANCE Expanded
Dataset
Demographics and Baseline
Characteristics
HVAD
Control
P-value
Age (years) 63.9 ± 11.6 66.2 ± 10.2 0.044
Male gender (%) 76.4% 82.4% 0.178
Race (%)
White
Black or African American
Other
76.8%
22.2%
1.0%
77.7%
21.6%
0.7%
0.962
Height (cm) 173.8 ± 9.4 175.5 ± 9.1 0.068
Body Surface Area (m
2
) 2.0 ± 0.3 2.0 ± 0.3 0.615
1
2
3
4
5-7
3.4%
29.0%
40.4%
19.9%
7.4%
3.4%
31.1%
40.5%
18.2%
6.8%
0.989
Ischemic Etiology of Heart Failure 57.9% 60.1% 0.684
Smoker 68.0% 62.2% 0.243
Diabetic 44.4% 43.9%
Previous Stroke/TIA 19.2% 16.2% 0.515
Hypertension requiring medication 65.3% 70.9% 0.241
Serum creatinine (mg/dL) 1.5 ± 0.5 1.4 ± 0.5 0.760
12.0%
5.5%
0.040
Left ventricular ejection fraction (LVEF, %) 17.1 ± 4.6 16.2 ± 4.8 0.055
below in Figure 7. The expanded dataset includes a higher proportion of HVAD devices
Supplemental) to pooled Control subjects were also performed, as shown in Figure 7.
 Loading...
Loading...