34 HVAD® Instructions for Use
10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
Pump Overview
Introduction
1.8 US Clinical Study: Destination Therapy (continued)
Device Failures and Malfunctions
The incidence of device failures and device malfunctions within 730 days was 31.4% in the
HVAD arm vs. 25.5% in the Control arm. The rates of pump thrombosis were similar in both arms,
though sintering of the HVAD did appear to decrease this event. Device malfunctions related to
controller faults were substantially more frequent in the HVAD arm.
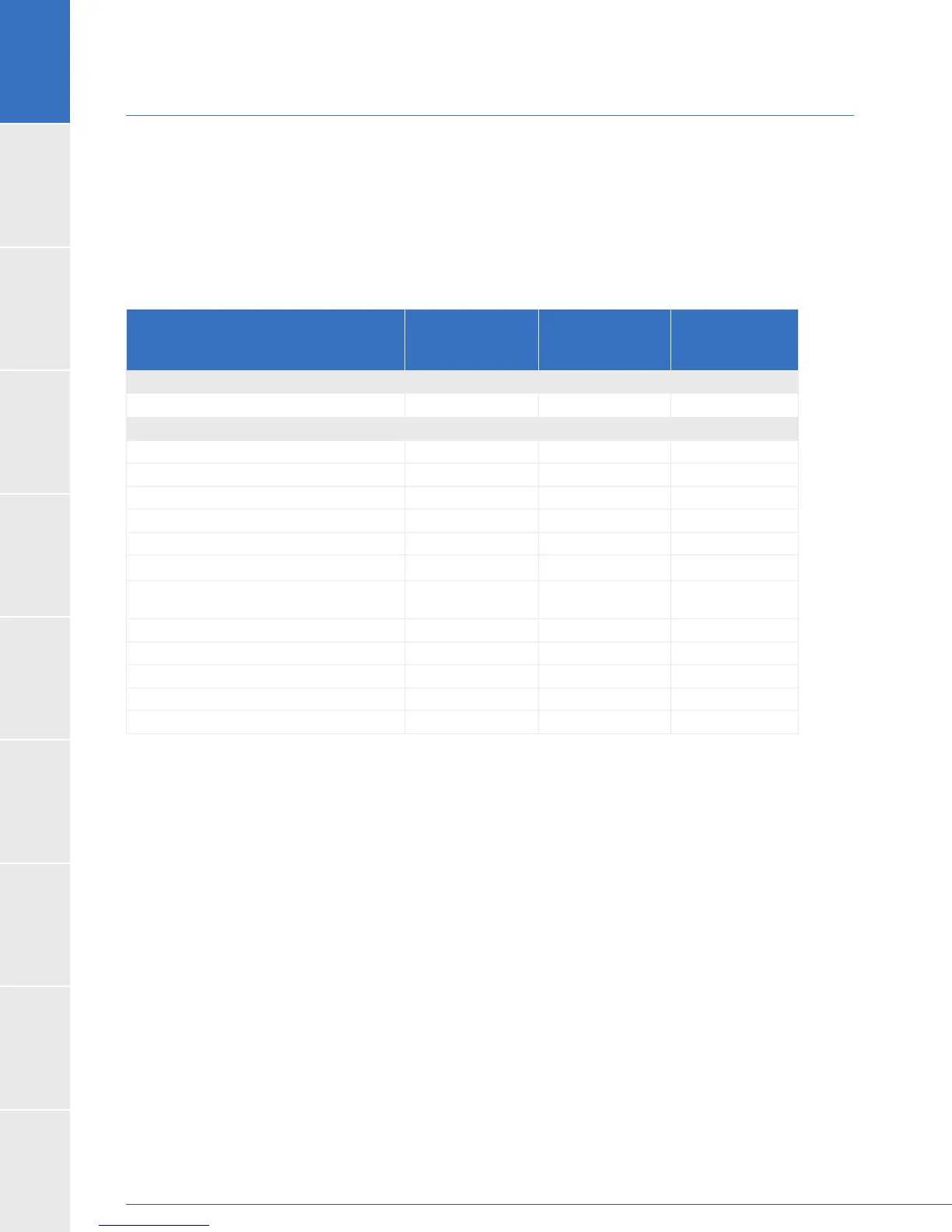
Table 17: Device Failure or Malfunctions in the ENDURANCE Trial Expanded Dataset
Parameter
HVAD
Sintered
HVAD
Control
Based on CEC Adjudication Data
Device Failure 30.5% (6) 33.3% (32) 25.5% (38)
Type of Device Malfunction
Controller fault 10.0% (20) 7.3% (7) 2.7% (4)
Critical low battery 0.0% (0) 1.0% (1) 0.7% (1)
Damaged battery 1.0% (2) 0.0% (0) 0.0% (0)
Damaged cable 2.5% (5) 3.1% (3) 4.0% (6)
Damaged controller 2.0% (4) 3.1% (3) 0.0% (0)
Electrical fault 2.0% (4) 0.0% (0) 0.0% (0)
Iatrogenic/Recipient-Induced
Failure
0.5% (1) 0.0% (0) 0.7% (1)
1.5% (3) 1.0% (1) 0.0% (0)
Power disconnect 2.5% (5) 0.0% (0) 1.3% (2)
Pump 0.0% (0) 0.0% (0) 2.7% (4)
Pump Thrombosis 10.0% (20) 22.9% (22) 11.4% (17)
Other 4.5% (9) 1.0% (1) 3.4% (5)
Rehospitalizations
The average number of re-hospitalizations within 730 days after the initial hospitalization
was similar between the HVAD arm and the Control arm, as shown in Figure 11. For the AIP
population, the HVAD subjects were re-hospitalized on average, 4.1 times, compared to 3.6
times in the Control group.
 Loading...
Loading...