10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
33Introduction
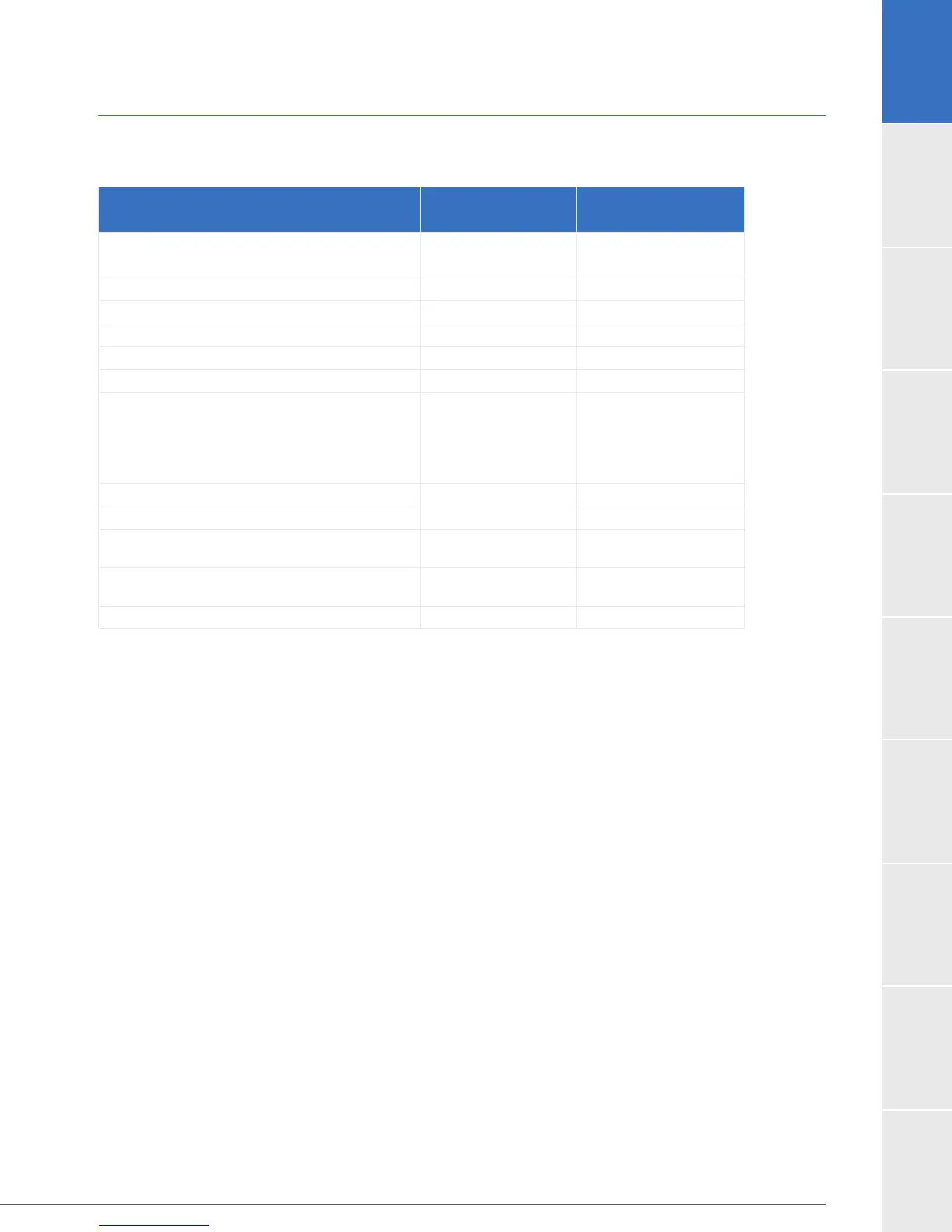
1.8 US Clinical Study: Destination Therapy (continued)
Table 16: Summary of INTERMACS Adverse Events Occurring Through 2 Years in Subjects in the
ENDURANCE Trial Expanded Dataset
Adverse Event
HVAD
Control
Overall Bleeding events
GI Bleed
60.1% (178)
35.1% (104)
60.4% (90)
34.2% (51)
Cardiac Arrhythmia 37.8% (112) 40.9% (61)
Hepatic Dysfunction 4.7% (14) 8.1% (12)
Hypertension 15.9% (47) 16.8% (25)
Sepsis 23.6% (70) 15.4% (23)
Driveline Exit Site Infection 19.6% (58) 15.4% (23)
Stroke
Ischemic Cerebrovascular Event
Hemorrhagic Cerebrovascular Event
TIA
29.7% (88)
17.6% (52)
14.9% (44)
8.4% (25)
12.1% (18)
8.1% (12)
4.0% (6)
4.7% (7)
Renal Dysfunction 14.9% (44) 12.1% (18)
Respiratory Dysfunction 29.1% (86) 25.5% (38)
Right Heart Failure
38.5% (114)
2.7% (8)
26.8% (40)
3.4% (5)
Pump Replacement
Exchange for Pump Thrombosis
7.8% (23)
6.4% (19)
13.4% (20)
10.7% (16)
Device Malfunction or Failure 31.4% (93) 25.5% (38)
*Site-reported event.
Abbreviations:
Note:
Stroke-related Deaths
Per CEC adjudication, among the full AIP population 12.5% (37/296) of HVAD patients and
6.7% (10/149) of Control patients had stroke-related deaths (data lock date of May 30, 2017, all
of death from stroke that was 87% greater than the risk of Control patients. The rate of stroke-
related death within 2 years of implantation was 8.4% (25/296) for HVAD patients and 6.0%
(9/149) for Control patients. The rate of later-onset stroke-related death (i.e., stroke occurring
after 2 years of LVAD support) was 3.7% (11/296) for HVAD patients and 0.7% (1/149) for Control
patients. The majority of HVADs which were involved with stroke-related deaths had sintered inlet
cannulae.
 Loading...
Loading...