36 HVAD® Instructions for Use
10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
Pump Overview
Introduction
1.8 US Clinical Study: Destination Therapy (continued)
Figure 13:
Quality of Life
The quality of life was assessed by the EQ-5D-5L and the KCCQ questionnaires, as summarized
in Figure 14. At baseline, subjects in both cohorts had poor quality of life and health status
assessed by KCCQ and EuroQOL EQ-5D. At 3 months, median KCCQ score had improved by
27.3 points and 24.2 points for study and control subjects, respectively. EuroQOL EQ-5D VAS
improved an average of 1.6 points at 3 months for subjects in the study arm and 1.7 points at
3 months for subjects in the control arm. Improvements in KCCQ and EuroQOL EQ-5D were
sustained during the follow-up period.
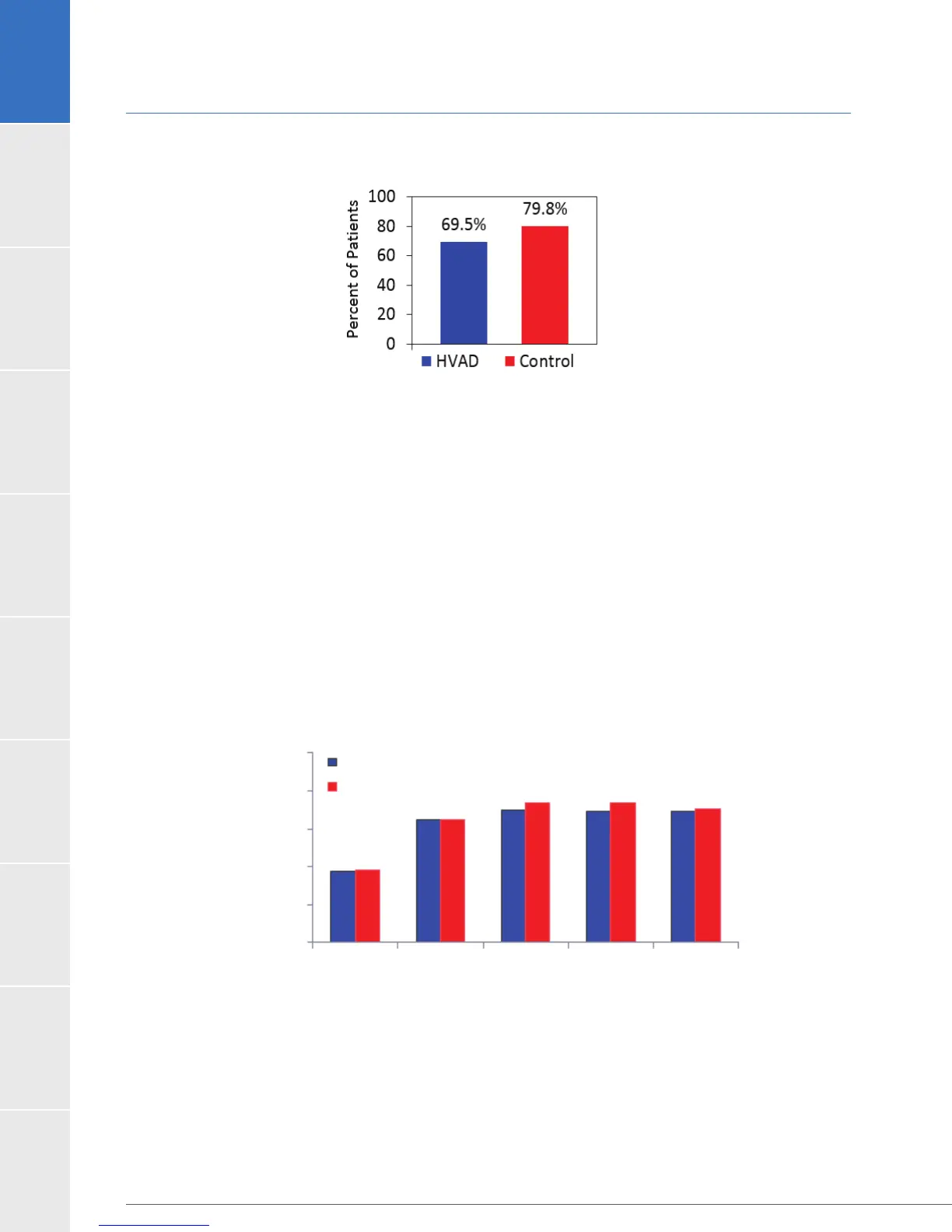
Figure 14:
Dataset. A) Change over time of the KCCQ Overall Summary Score. B) Change over baseline in the EQ-5D
Visual Analog Scale.
A. KCCQ
3 monthBaseline
HVAD
Control
6 month 12 month 24 month
100.0
80.0
60.0
40.0
20.0
0.0
Overall Summary Score
 Loading...
Loading...