10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
37Introduction
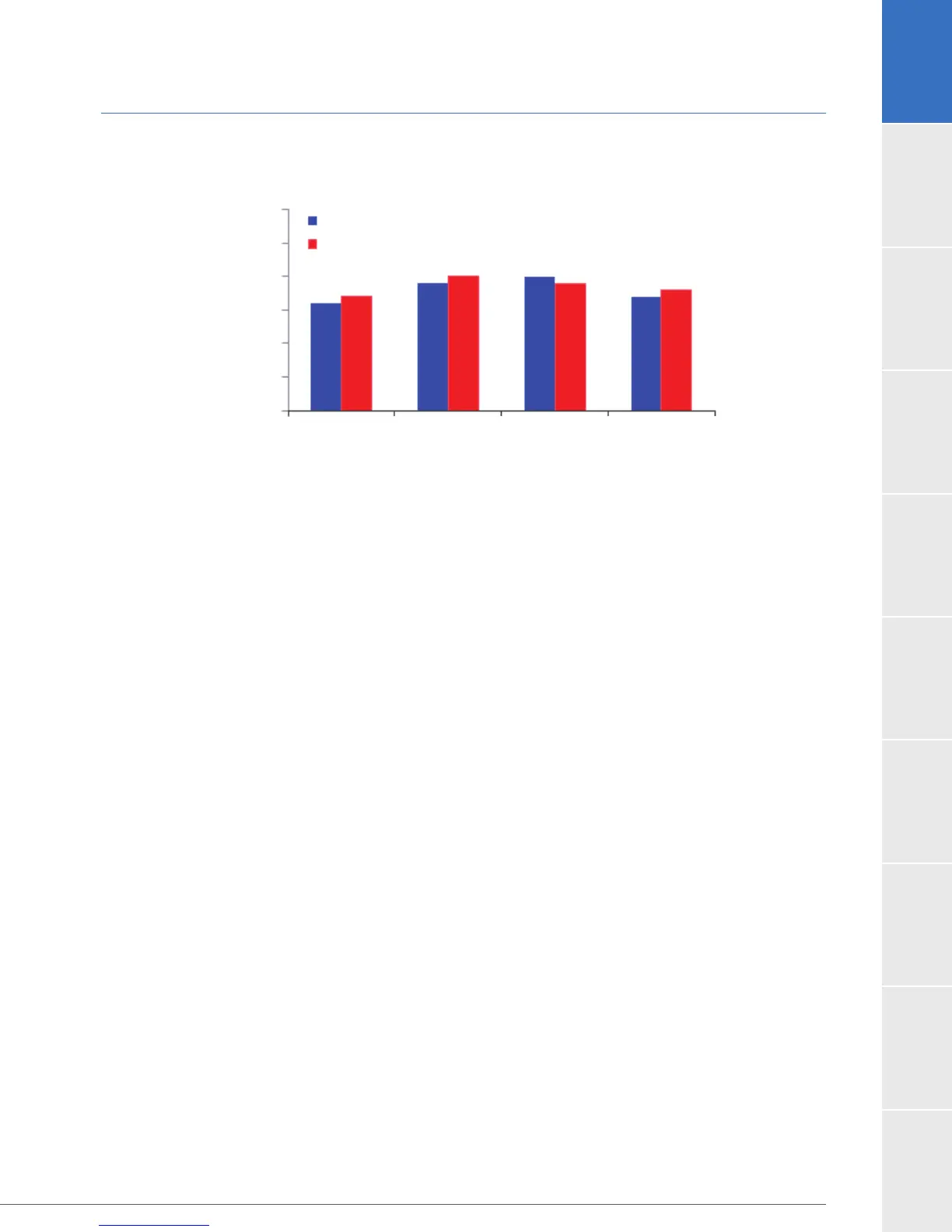
1.8 US Clinical Study: Destination Therapy (continued)
B. EQ-5D
3 months
HVAD
Control
6 months 12 months 24 months
3.0
2.5
2.0
1.5
1.0
0.5
0.0
Change from Baseline
Subgroup Analyses
The following preoperative characteristics were evaluated for potential association with
outcomes: gender, and BSA (< 1.5 m
2
2
no major clinical differences in outcomes based on gender or BSA.
Conclusions from the ENDURANCE Destination Therapy Trial
inferiority of the HVAD to the control device for patients alive on original device at two (2) years
dataset demonstrated similar endpoint results, with 57.4% success for control and 55% success
for HVAD. Following LVAD implant, approximately 80% of subjects in both arms improved to
distance increased in both arms (210 meters and 201 meters for study and control subjects,
respectively). Patients in both arms also showed comparable improvement in quality of life
from baseline to 3 months as measured by EQ-5D-5L and KCCQ, and the results were sustained
through 2 years.
 Loading...
Loading...