10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
PumpOverviewIntroduction
45Introduction
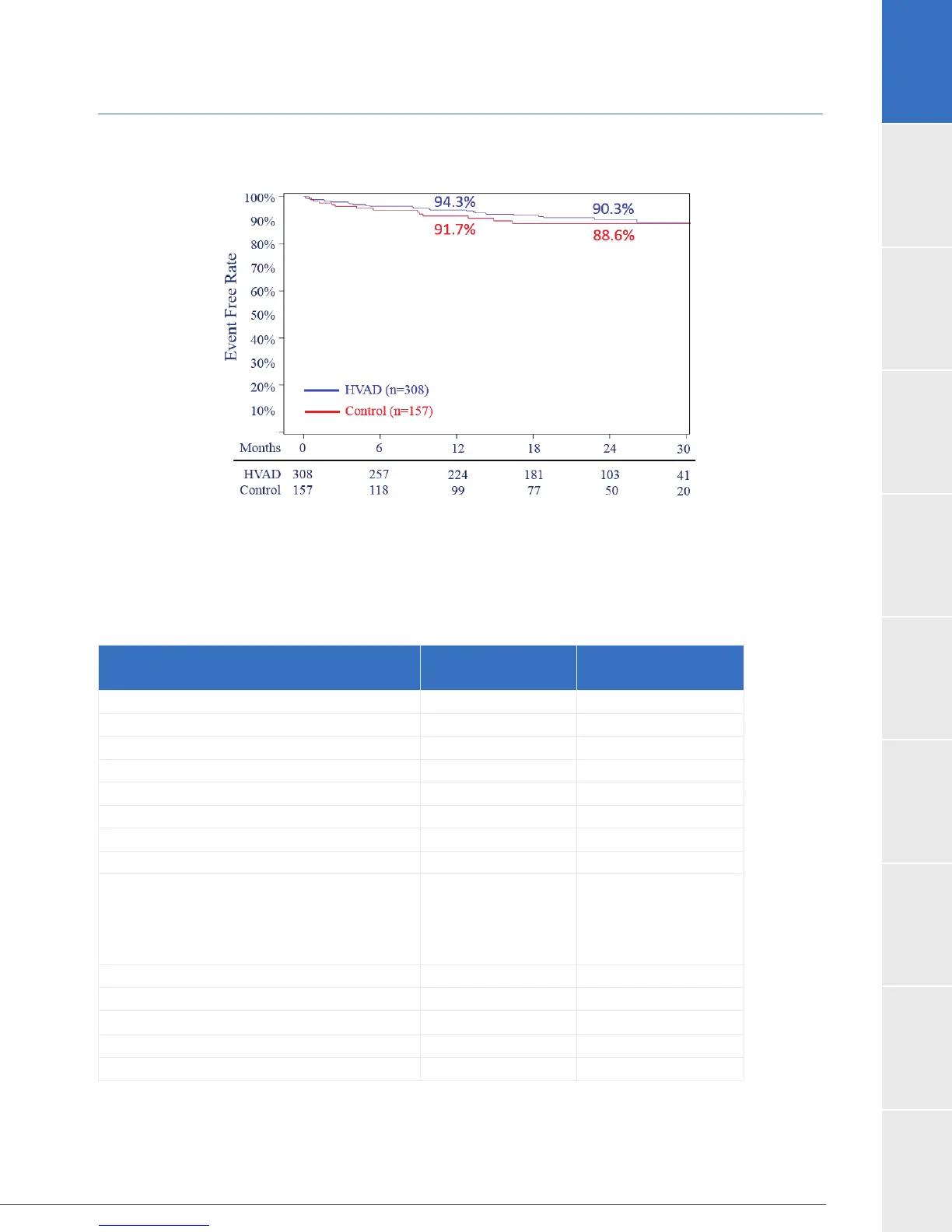
1.9 Destination Therapy Supplemental Study (continued)
Figure 20:
3. Adverse Events
Table 20 lists all the adverse events that occurred in the safety cohort.
Table 20: Summary of Adverse Events at 1 Year in the ENDURANCE Supplemental Trial.
Adverse Event
HVAD
Control
Major Bleeding 51.6% (159) 56.7% (89)
Cardiac Arrhythmia 34.1% (105) 31.2% (49)
Hepatic Dysfunction 3.9% (12) 3.8% (6)
Hypertension 13.0% (40) 12.7% (20)
Major Infection 53.9% (166) 59.2% (93)
Driveline Exit Site Infection 16.2% (50) 12.1% (19)
Device Malfunction/Failure 24.0% (74) 24.2% (38)
Hemolysis 1.3% (4) 5.7% (9)
Stroke
Ischemic Cerebrovascular Event
Hemorrhagic Cerebrovascular Event
TIA
16.9% (52)
13.0% (40)
5.2% (16)
4.2% (13)
14.6% (23)
7.6% (12)
7.0% (11)
0.6% (1)
Renal Dysfunction 10.4% (32) 14.6% (23)
Respiratory Failure 19.8% (61) 19.7% (31)
Right Heart Failure 35.4% (109) 38.2% (60)
Pump Replacement 5.2% (16) 11.5% (18)
Exchange for Pump Thrombosis 4.5% (14) 10.2% (16)
 Loading...
Loading...