46 HVAD® Instructions for Use
10
9
8
7
6
5
4
3
2
1
Appendix
Reference
Guides
Alarms and
Emergencies
Patient
Management
Surgical
Implant and
Explant
Monitor
Peripherals
and
Accessories
HVAD
®
Pump Overview
Introduction
1.9 Destination Therapy Supplemental Study (continued)
Stroke-related Deaths
Within the mITT population, the CEC-adjudicated rate of stroke-related death within 1 year of
implantation was 3.2% (10/308) for HVAD patients and 2.5% (4/157) for Control patients.
related death decreased by the same proportions (approximately 58%) for both HVAD and
Control arms; only the HVAD arm was exposed to the trial’s investigational intervention of a
blood pressure management protocol. The stroke-related deaths are compared in Table 21.
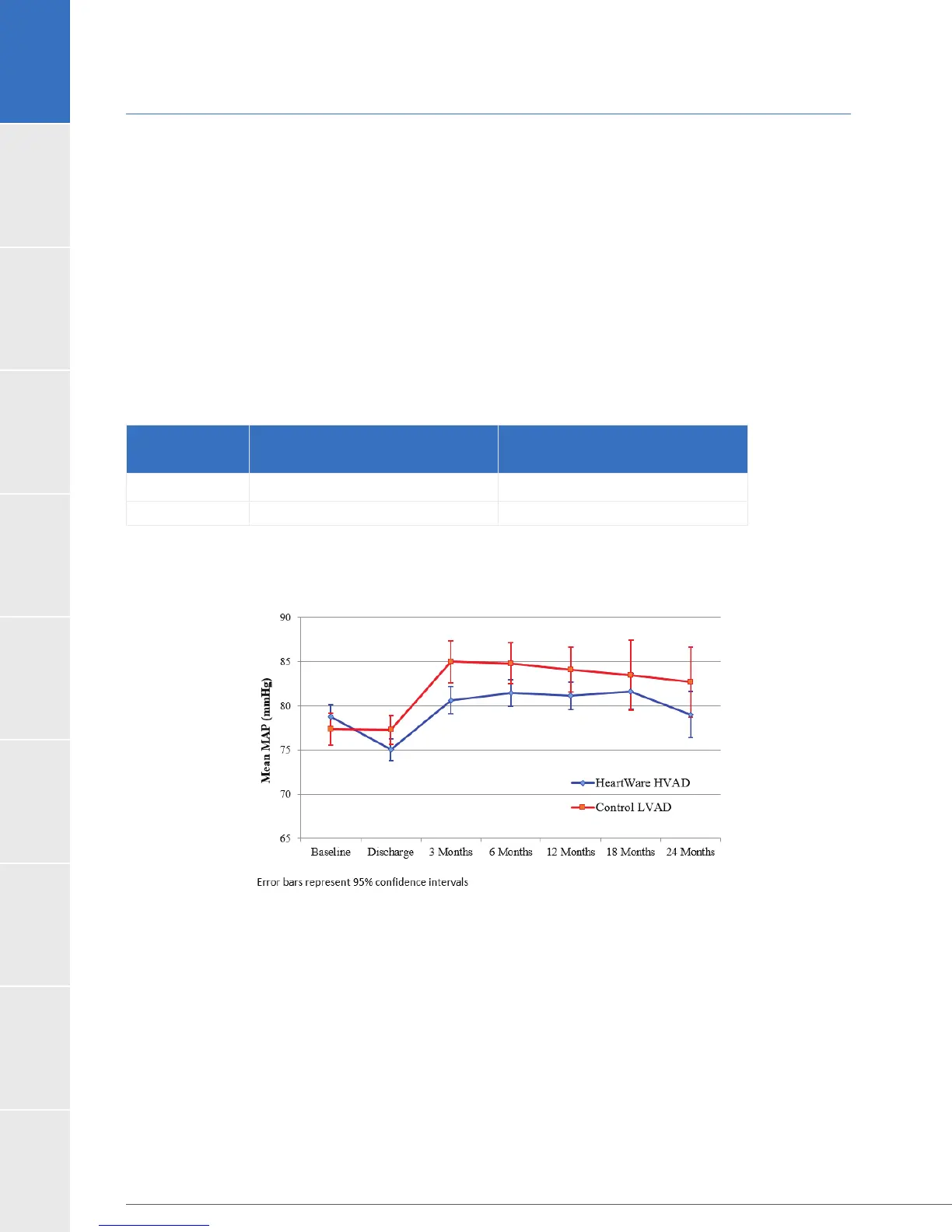
Control is shown in Figure 21.
Table 21: Stroke-related Deaths in ENDURANCE and ENDURANCE Supplemental Trials
Within 2 years of implant (AIP)
Within 1 year of implant (mITT)
HVAD
25/296 (8.4%) 10/308 (3.2%)
HMII (control)
9/149 (6.0%) 4/157 (2.5%)
Figure 21:
Health Status and Functional Improvements
The improvements in quality of life, as measured by the KCCQ and EQ-5D-5L, and functional
Figure 22.
 Loading...
Loading...