Computing Absorbance Data Points 51
4
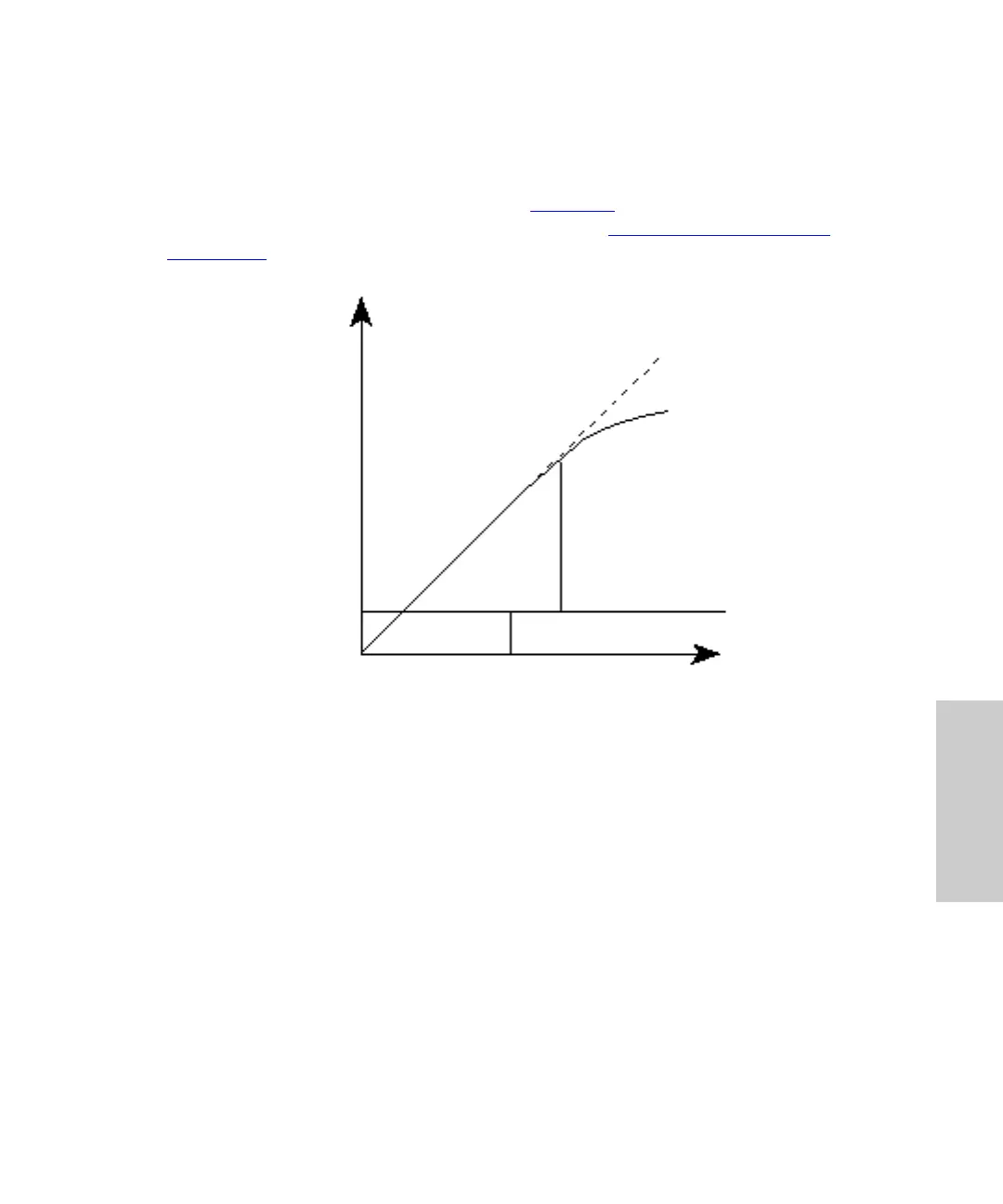
Beer’s Law applies only to well-equilibrated dilute solutions. It assumes that the refractive
index of the sample remains constant, that the light is monochromatic, and that no stray
light reaches the detector element. As concentration increases, the chemical and
instrumental requirements of Beer's law may be violated, resulting in a deviation from
(absorbance versus concentration) linearity (Figure 4-4
). The absorbance of mobile phase
can reduce the linear range by the amounts shown in Appendix D,
Mobile Phase
Absorbance.
Figure 4-4 Absorbance as a Function of Concentration
Dark Current
Photodiodes lose charge over time even when they are not exposed to light. The amount
of charge lost is called
dark current
.
At the start of a chromatographic run, the 996 detector closes the shutter to take a dark
current reading for each diode. The shutter closes after the exposure time is calculated
and stays closed for the same interval as the exposure time.
The detector subtracts the dark current values from the current values recorded during
absorbance measurements for both the sample and the reference spectra.
Working Range
AbsorbanceBackground
Concentration
Absorbance
Ideal
Actual
 Loading...
Loading...