1.3.1 EU Directives
Introduction
This section describes the EU Directives that apply to the Biacore X100 instrument.
Conformity with EU Directives
This product fulfills the European Directives listed below. See the EU Declaration of
Conformity for the directives and regulations that apply for the CE marking.
If not included with the product, a copy of the EU Declaration of Conformity is available
on request.
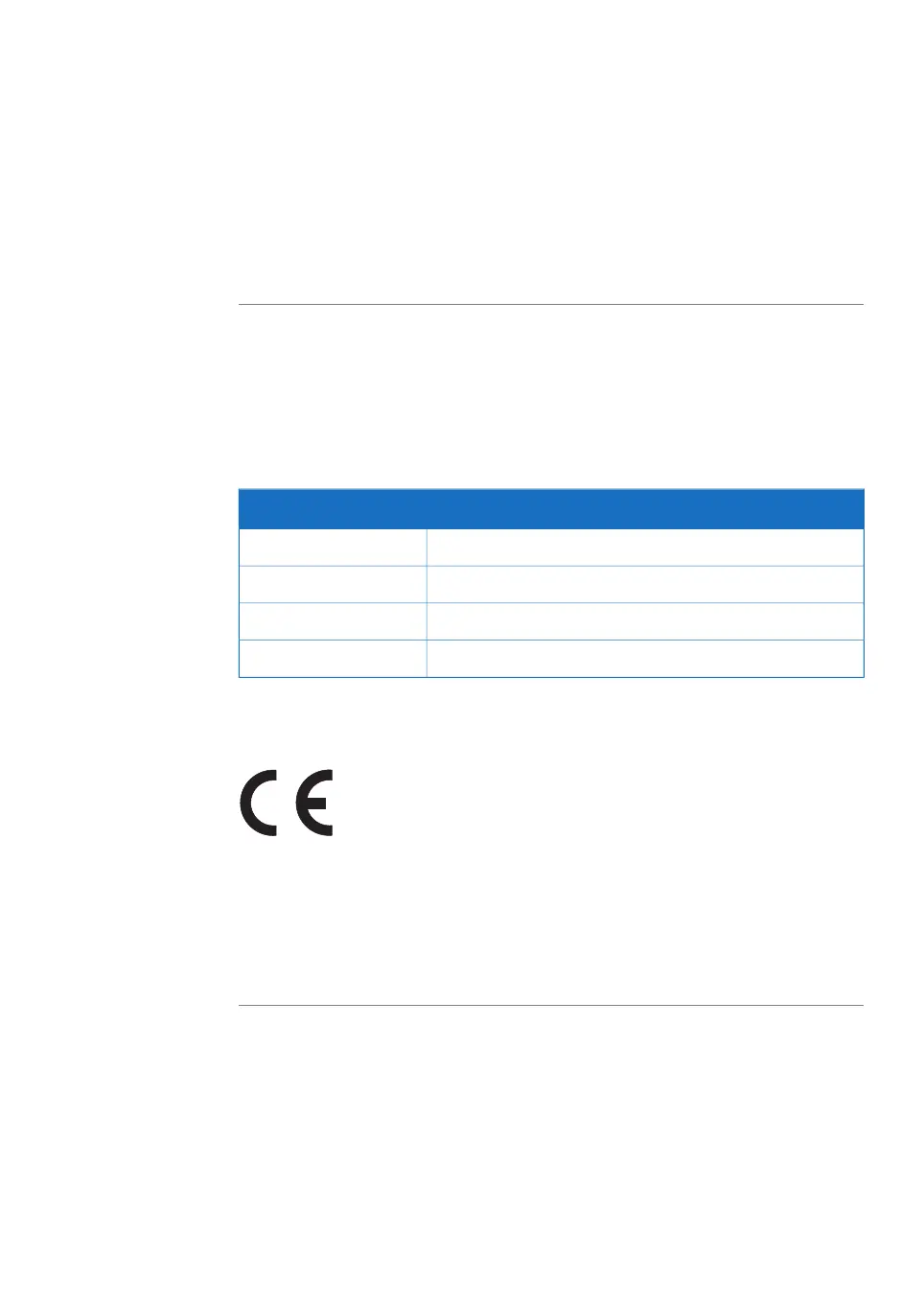
TitleDirective
Machinery Directive (MD)2006/42/EC
Electromagnetic Compatibility (EMC) Directive2014/30/EU
Low Voltage Directive (LVD)2014/35/EU
Restriction of Hazardous Substances (RoHS) Directive2011/65/EU
CE marking
The CE marking and the corresponding EU Declaration of Conformity is valid for the in-
strument when it is:
•
used according to the Operating Instructions or user manuals, and
•
used in the same state as it was delivered from GE, except for alterations described
in the Operating Instructions or user manuals.
Biacore X100 Operating Instructions 28961142 AD 9
1 Introduction
1.3 Regulatory information
1.3.1 EU Directives

 Loading...
Loading...