D-0126445-A – 2020/08
VisualEyes™ 515/525 Instructions for Use - EN Page 2
2 Unpacking and inspection
2.1 Unpacking and inspection
Check shipping containers and contents for damage
When the VisualEyes™ system is received, please check the shipping box for rough handling and damage.
Ensure that you have received all the components on the shipping checklist. All the components should be
checked visually for scratches and missing parts before use. All the contents of the shipment have to be
checked for their mechanical and electrical functioning. If the equipment is found faulty, please contact your local
distributor immediately. Keep the shipping materials for the carrier’s inspection and insurance claim.
Keep carton for future shipment
The system comes with shipping cartons, which are specifically designed for the components. It is
recommended to keep the cartons for future shipments in case of any need for return or service.
Reporting and returning procedure
Any missing part or malfunction should be reported immediately to the supplier/local distributor along with the
invoice, serial number and a detailed report of the issue. For any on-site service-related information, please contact
your local distributor. If the system / components are to be returned for service, please fill all the details related to
product issues in the ‘Return Report’, which is attached to this manual. It is very important that you describe all
the known facts about the issue in the return report, as this will help the engineer to understand and solve the
problem to your satisfaction. Your local distributor holds the responsibility for coordinating any service/return
procedure and related formalities.
2.2 Marking
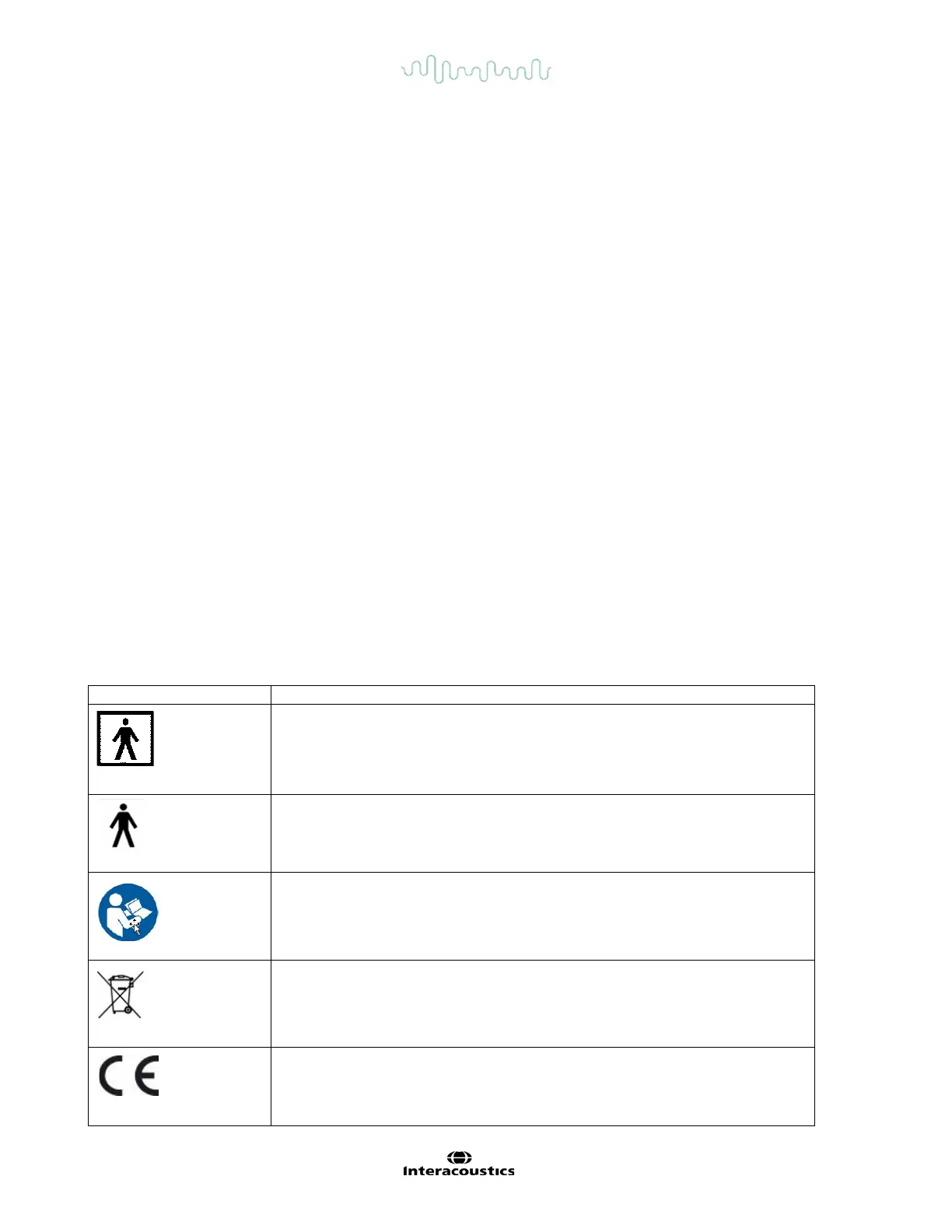
The following markings (Table 2.2-1) can be found on the equipment:
Table 2.2-1 Label markings and Explanations for VisualEyes
TM
Follow instructions for use
This symbol indicates that when the end-user wishes to discard this product,
it must be sent to separate collection facilities for recovery and recycling.
Failing to do so may endanger the environment.
0123
The CE-mark indicates that Interacoustics A/S meets the requirements of
Annex II of the Medical Device Directive 93/42/EEC. TÜV Product Service,
Identification No. 0123, has approved the quality system

 Loading...
Loading...











