PASCAL
®
Synthesis Ophthalmic Scanning Laser Systems Page 108 of 141
88292-EN, Rev D
PASCAL Synthesis
(532 nm)
PASCAL Synthesis
(577 nm)
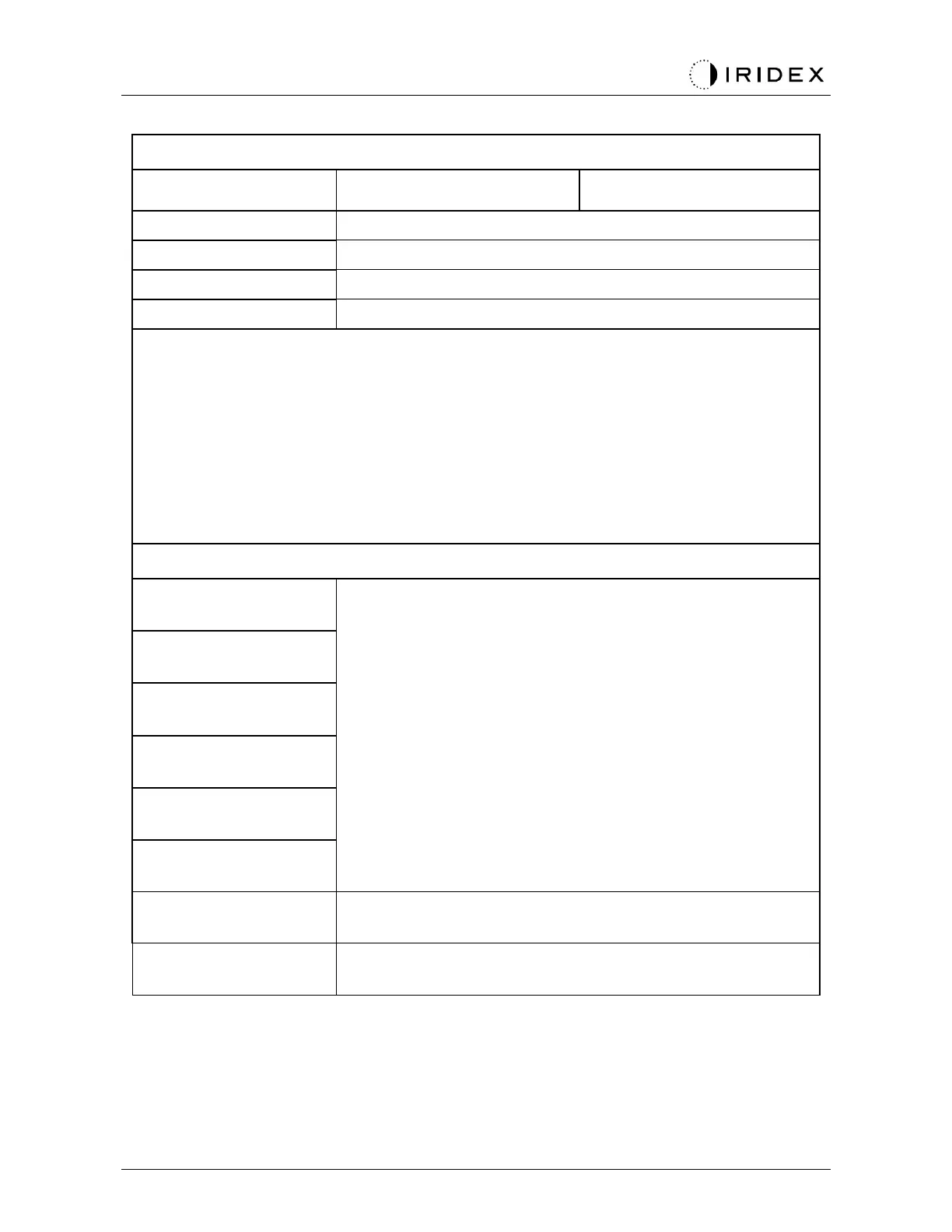
Product Classifications per IEC 60601-1
Standard equipment, Footswitch is IPX1
Equipment not suitable for use in the presence of a flammable anesthetic mixture with
air or with oxygen or nitrous oxide
Classifications & Approvals
Laser Safety Requirements for Diagnostic and Therapeutic
Laser Equipment
International Safety Requirements for Medical Electrical
Equipment
EMC Requirements for Medical Electrical Equipment
Risk Management for Medical Devices
CAN/CSA-C22.2 No.
60601-1
Canadian deviations for Medical Electrical Equipment
US Safety Requirements for Medical Electrical Equipment
Tested and Complies With FCC Part 15 Class B
 Loading...
Loading...