Page 5
Safety notes 1
Operating Instruction Responsibility of the user 1.1
Art. no.: 2.510474 rev.: d
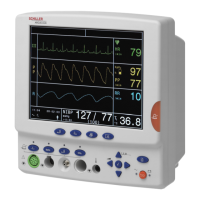
ARGUS LCM/PLUS
1 Safety notes
1.1 Responsibility of the user
1.2 Intended use
1.3 Organisational measures
V This device must only be used by qualified doctors or trained medical personnel
under their direct supervision.
V The numerical and graphical results and any interpretation given must be exam-
ined with respect to the overall clinical condition of the patient and the general re-
corded data quality.
V The indications given by this equipment are not a substitute for regular checking
of vital functions.
V Specify the competencies of the personnel for operation and repair.
V Ensure that the personnel have read and understood these operating instructions
and in particular this chapter “safety notes".
V Have damaged or missing components replaced immediately.
V The operator is responsible for compliance with all applicable accident prevention
and safety regulations.
V The ARGUS LCM/PLUS is a patient monitoring device used for the measuring of
the parameters of a patient, including ECG, SpO
2
, CO
2,
non invasive blood pres-
sure, temperature and respiration.
V The system is suitable for internal hospital transport.
V There is no danger for patients with pacemaker.
V The device is only intended for single patient use.
V Only operate the device in accordance with the specified technical data.
V The system is not designed for sterile use nor is it designed for outdoor use.
V Do not use this unit in areas where there is any danger of explosion or in the pres-
ence of flammable gases such as anaesthetic agents.
V This unit is CF classified. It is defibrillation protected only when the
SCHILLER original patient cable is used. However, as a safety precaution when
possible, remove electrodes before defibrillation.
V This product is not designed for direct cardiac application.
V Before using the unit, ensure that an introduction regarding the unit functions and
the safety precautions has been provided by a medical product representative.
V Keep these operating instructions in an accessible place for reference when re-
quired. Make sure that they are always complete and legible.
V Observe the operating instructions and maintenance instructions.
V These operating instructions do not override any statutory or local regulations, or
procedures for the prevention of accidents and environmental protection.
 Loading...
Loading...