Appendix F Acoustic Output Reference
F - 10 Instructions for Use
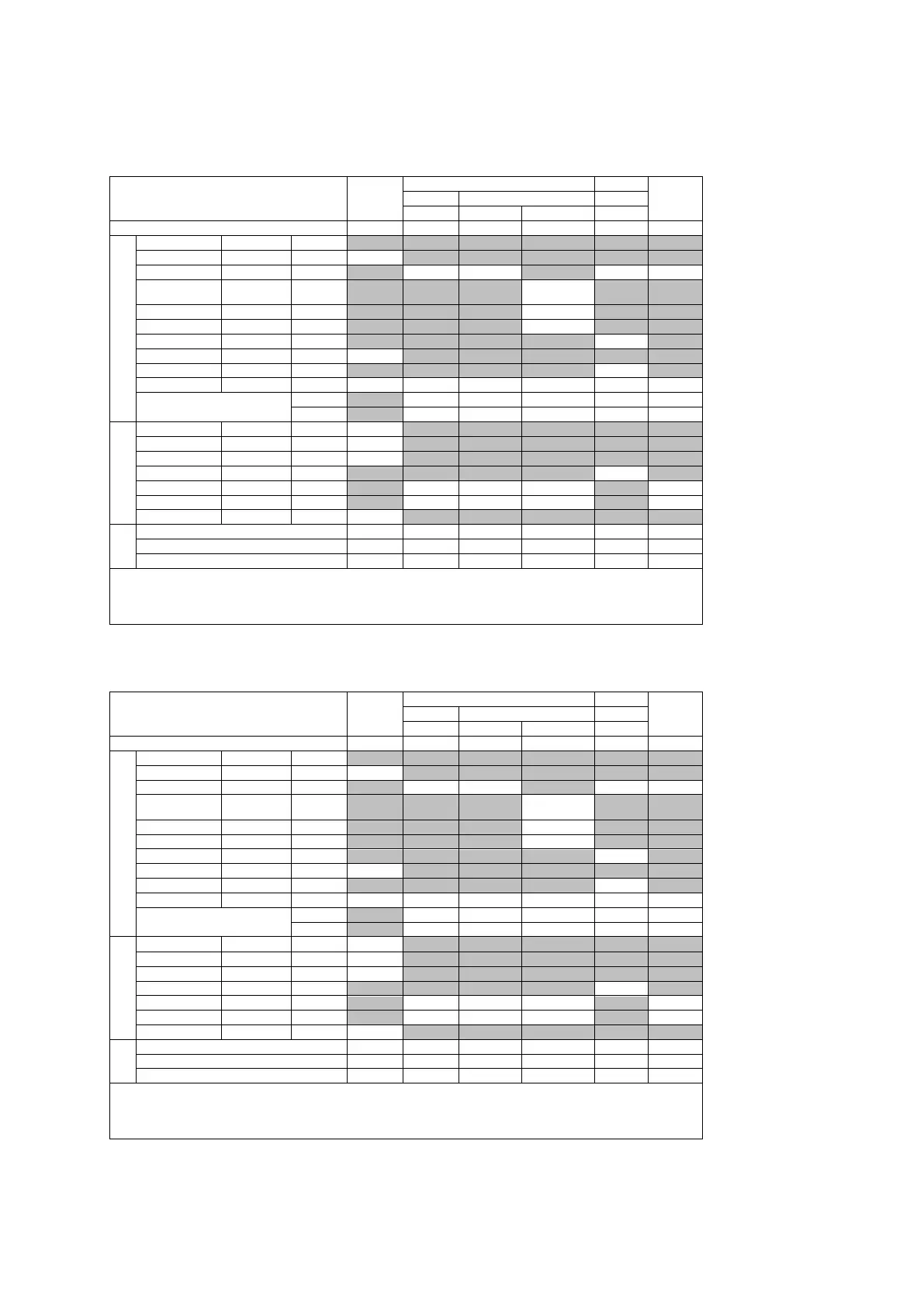
Acoustic Output Reporting Table – Track 3, FDA 510(k) and IEC 60601-2-37
(Per transducer/mode that exceeds MI or TI value of 1.0)
Transducer model: 4C1 Operating mode: 2D-Mode
A
aprt
>1
Associated Acoustic Parameters
.3
1
I
(z
)]
α
s
I
(z
)]
(mW)
-
aprt
Other Information
PD t
d
(µsec)
1.27
FL
Control
a This Index is not relevant to this operating mode.
b This transducer is not intended for transcranial or neonatal cephalic uses.
c This formulation for TIS is less than that for an alternate formulation in this mode.
# No data is provided for this operation condition since the maximum index value is not reported for the reason listed.
Acoustic Output Reporting Table – Track 3, FDA 510(k) and IEC 60601-2-37
(Per transducer/mode that exceeds MI or TI value of 1.0)
Transducer model: 4C1 Operating mode: 2D-Mode (THI)
A
1
A
aprt
>1
Associated Acoustic Parameters
p
.3
1
I
TA.3
(z
1
)]
α
s
I
ta.α
(z
s
)]
-
z
Dim. of A
aprt
Other Information
PD t
d
(µsec) 0.79
Control
a This Index is not relevant to this operating mode.
b This transducer is not intended for transcranial or neonatal cephalic uses.
c This formulation for TIS is less than that for an alternate formulation in this mode.
# No data is provided for this operation condition since the maximum index value is not reported for the reason listed.

 Loading...
Loading...