40
Operating Instruction TNI soft Flow 50 homecare system
7) Technical data
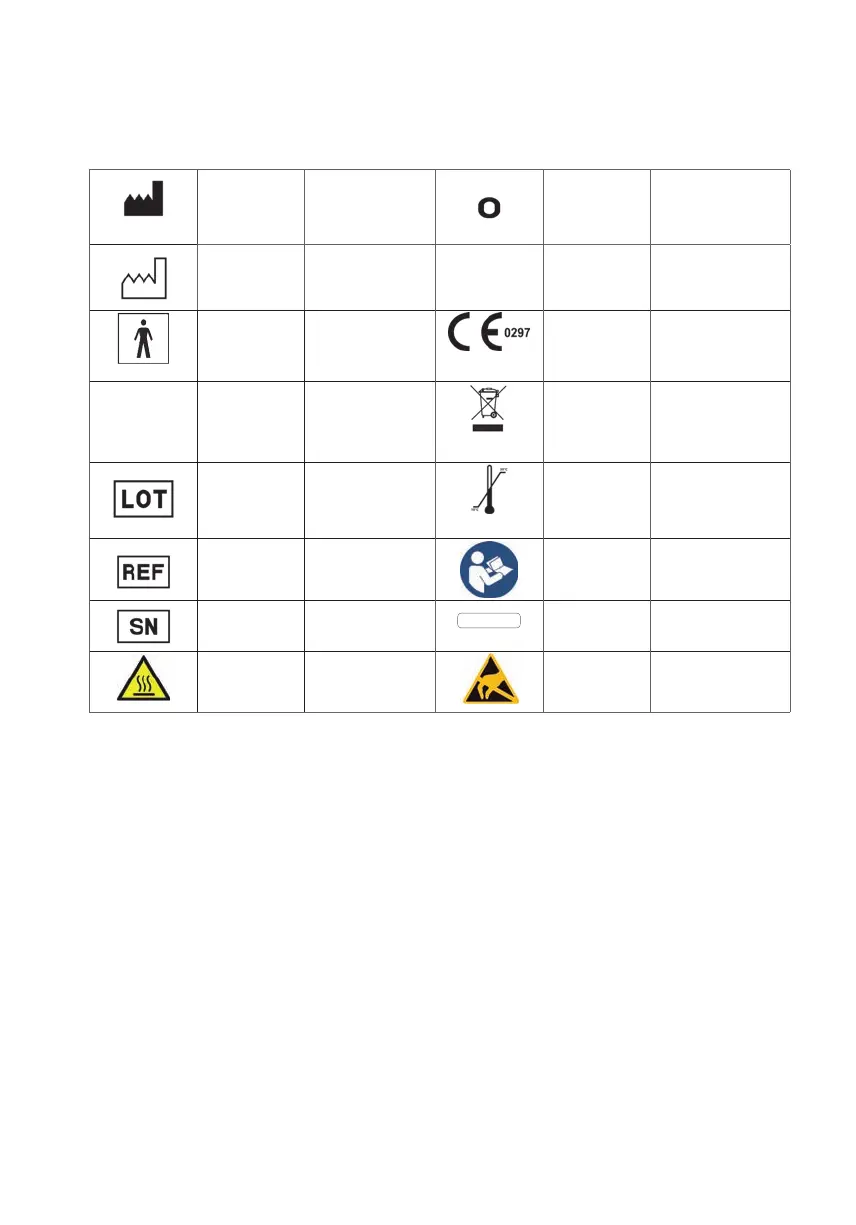
7.1) Symbols on the device
Manufacturer
TNImedicalAG
Hofmannstraße8
D-97084Wuerzburg
Phone:+4993120792902
Fax:+4993120792918
Email:info@tni-medical.de
Powerswitch:OFF
Ontheswitch
Thedeviceisdisconnectedfrom
powersupply
Productiondate
Indicatesthedatewhenthemedi-
calproductwasproduced
I
Powerswitch:ON
Ontheswitch
Thedeviceisconnectedwithpower
supply
Appliedpartof
typeBF
Protectiondegreeforapplied
part:BF
Theuserissowellisolatedby
thedevicethatthesafetyregula-
tionsconcerningleakagecurrent
aremet.
CEmark
Typelabel
Conrmsconformitywiththeregula-
tion93/42/EWGofmedicalproducts
IP 21
IP-protectionclass
Protectedagainstsolidforeign
objectsof12.5mmandgreater.
ProtectedagainstDroptfrom
above
Disposal
Typelabel
TheTNI soft Flow 50mustnotbe
disposedofinhouseholdwaste.
Accordingtothenewelectricand
electronicequipmentlaw(ElektroG)
themanufacturerisresponsiblefor
thedisposalofTNI soft Flow 50
.PleasecontactTNImedicalAG
regardingtheunit`sdisposal.
Batchcode
Indicatesthebatchcodeofthe
manufacturer,forthatthebatchor
dasLoscanbeidentied
Ambient
temperature
Thedevicemayonlybeoperated
withtheambientconditions
Item/article
number
Indicatestheordernumberofthe
manufacturer,forthatthemedical
productcanbeidentied.
Attentiontothe
usermanual
(operating
instructions)
Attentiontotheusermanualor
pleasenoteoperatinginstructions
Serialnumber
Indicatestheserialnumberofthe
manufacturer,forthataparticular
medicalproductcanbeidentied.
max. 200 mbar
Max.pressure
Max.pressureallowedattheoxy-
genintake
Caution:
hotsurface
Thesomarkeddevicepartsmay
gethotsurface
Heatingplate
Homecarechamber
Humidicationchamberautoll
Caution
Electrostatic
sensitive
ObservePrecautionsforHandling
electrostaticsensitiveDevices
 Loading...
Loading...