Safety
4-32
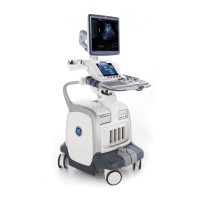
LOGIQ E9
–
User Guide
Direction 5454884-100 English
Rev. 1
Acceptable Devices
The Patient Environmental devices shown on the previous page
are specified to be suitable for use within the PATIENT
ENVIRONMENT.
Unapproved Devices
Accessories, Options, Supplies
DO NOT connect any probes or accessories without approval
by GE within the PATIENT ENVIRONMENT.
See ‘Peripheral Update for EC countries’ on page 4-24 for
more information.
DO NOT use unapproved devices.
If devices are connected without the approval of GE, the
warranty will be INVALID.
Any device connected to the LOGIQ E9 must conform to one or
more of the requirements listed below:
1. IEC standard or equivalent standards appropriate to
devices.
2. The devices shall be connected to PROTECTIVE EARTH
(GROUND).
Unsafe operation or malfunction may result. Use only the
accessories, options and supplies approved or recommended
in these instructions for use.
 Loading...
Loading...