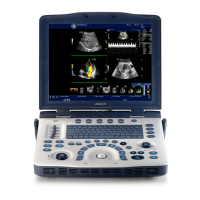
Overview
LOGIQ V2/LOGIQ V1 – User Guide 1-5
Direction 5610736-100 Rev. 9
Indications for Use
The LOGIQ V2/LOGIQ V1 is intended for ultrasound imaging,
measurement and analysis of the human body for multiple
clinical applications including: Fetal/OB; GYN; Abdominal;
Pediatric; Small Organ (breast, testes, thyroid); Neonatal and
Adult Cephalic; Cardiac (adult & pediatric); Peripheral Vascular;
Musculoskeletal Conventional & Superficial; Urology;
Transrectal; Transvaginal; imaging guidance of interventional
procedures (e.g Nerve Block; Vascular Access; Tissue Biopsy/
Fluid Drainage).
Frequency of Use
Daily (Typically 8 hours)
Operator Profile
• Qualified and trained physicians or sonographers with at
least basic ultrasound knowledge.
• The operator must have read and understood the user
manual.
NOTE: Only qualified physicians or sonographers should perform
ultrasound scanning on human subjects for medical diagnostic
reasons. Request training, if needed.
 Loading...
Loading...