15-17
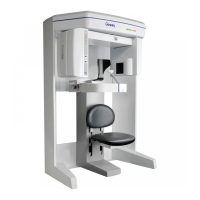
Radiation Environment Survey
G990710 30 June 2008
Recommended Operating Requirements
Local agencies or government bodies or international standards may
dictate requirements for installation of the system in order to protect
personnel and the public from exposure from the radiological output
of the device. Consult your local agencies, government bodies, or
international standards for actual requirements which apply.
It is recommended that a qualified Physicist or Radiologist
determine where appropriate, the applicable lead shielding to be
installed in the area around the system equipment. Below are some
other common requirements that may apply to your location:
• The Computer Workstation and X-ray Operator should be
located behind a properly shielded permanent barrier. A
viewing window (or alternative method such as a mounted
mirror) should be present to enable the X-ray Operator to
view the Patient and operate the computer while the
exposure is present.
• Operators should consider the use of a lead apron to protect
the anatomical areas of the medical personnel working in
the areas exposed to radiation.
• The Operator Control Box and Acquisition Computer shall
be located within 1 meter [3.28 ft] from door. If not, an
interlocked door may be required.
• A room door may be required.
• Radiation warning signs may be required next to the
entrance to the room.
• A Warning light may be required by the entrance to the
room.
• A shielding plan should be performed where the system is
being installed. Some local agencies or government bodies
require that a shielding plan be conducted by a qualified
Physicist or Radiologist and a copy of the shielding plan
be submitted and approved prior to installation of the
system.
• An area radiation survey by a qualified physicist or
Radiologist may then be required within 30 days of initial
clinical use of the system. This survey may be required to be
submitted to the local agency or government body.
 Loading...
Loading...