Section 8 --- Electrical Systems - House
Cayman 2005
280
If the low cell does not come up after charging, this battery can damage the rest of the
battery bank and should be replaced. An accurate digital volt meter + - .5% will also give an
indicator of the battery’s state of charge. Another test is to place a specific load on the bat-
tery for a predetermined length of time equal to that particular battery’s rating. This
machine is usually an adjustable carbon pile that can vary the load being applied to the
battery(s) while monitoring voltage to see if they will perform to their specific rated
capacities.
NOTE:
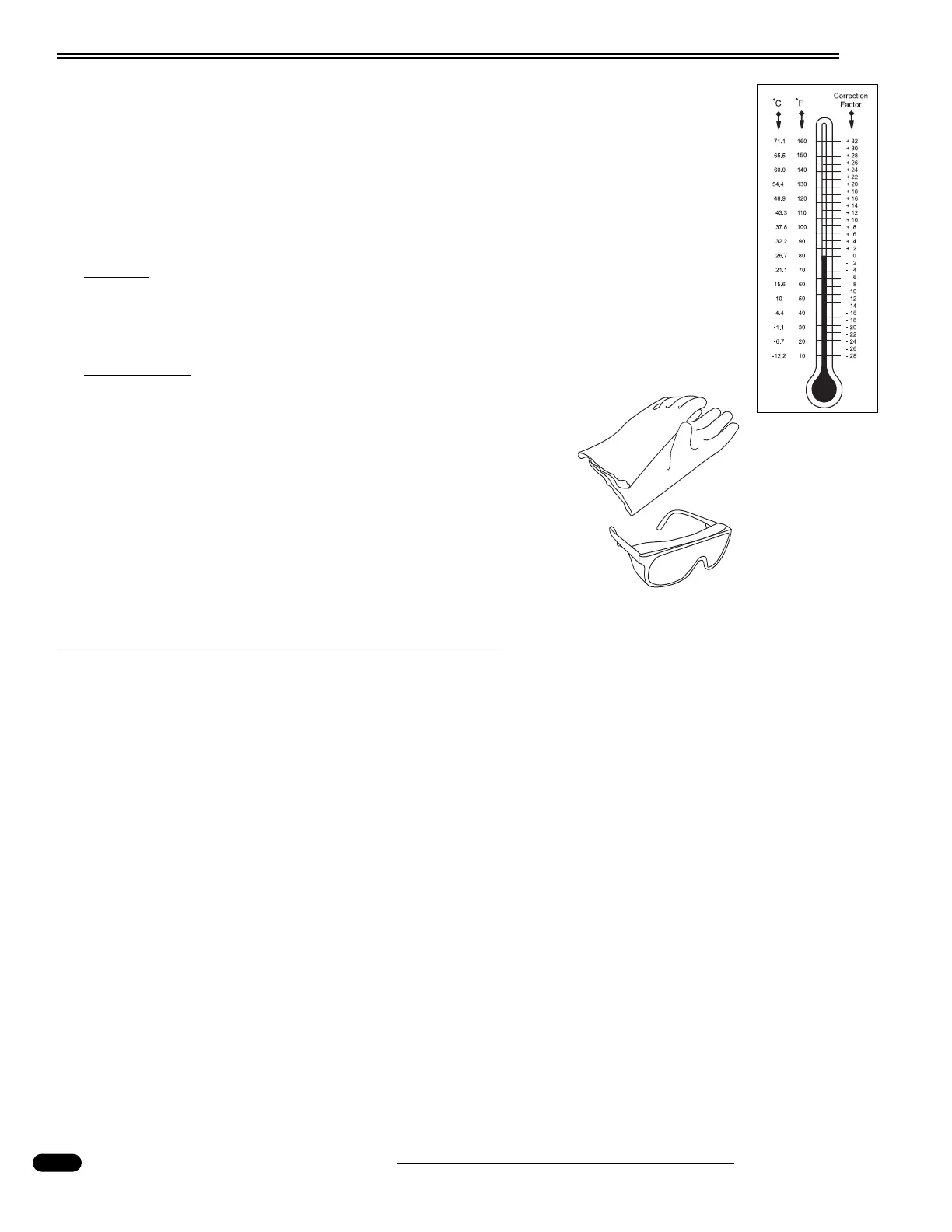
See the chart for temperature compensation. Liquid levels should be even
between the cells of the battery being tested as it will affect the accuracy of
the test.
W
ARNING:
Sulfuric acid in the batteries can cause severe injury
or death. Sulfuric acid can cause permanent damage
to eyes, burn skin and eat holes in clothing. Always
wear splash-proof safety goggles when working around
the battery. If the battery electrolyte is splashed in
the eyes, or on skin, immediately flush the affected
area for 15 minutes with large quantities of clean
water. In case of eye contact, seek immediate medical
aid. Never add acid to a battery once the battery has
been placed in service. Doing so may result in haz-
ardous splattering of electrolyte.
Battery Voltage & Current
Why does the voltage on a discharged battery measure the same as a fully charged battery until the
loads are applied? The simple answer is: A battery creates electrical power by converting energy from
a chemical reaction into electrical energy. As this reaction slows down the battery voltage will drop.
In a lead acid battery the electrolyte conductivity (how well electrical current can flow through it)
changes. The same current may be available but the rate of the reaction decreases, causing a voltage
drop.
Another way of looking at this is to use the analogy of a water pump (a battery is an electric pump).
The pressure in psi (pounds per square inch) that a pump delivers is like a battery’s voltage. The volume
of water in GPM (gallons per minute) is like the electrical current. Look at a 12 psi pump with no loads
(the pump is running but the outflow valve is turned off). The pump will run and the internal pressure
of the pump will build up to some point higher than 12 psi. When the valve is opened, and the water is
free to flow into the loads, the pressure will drop to the rated output pressure of 12 psi, but only if the
load is not too big. If the pump is designed to maintain 12 psi at 15 GPM, and a load demanding 20
GPM is connected, the pump will not be able to keep up and the pressure will drop to a lower psi. If
the load is reduced or removed the pump will catch up and return to its rated 12 psi pressure. If the
pump has an infinite source of water, such as a lake or the water utility (this is like the grid, no bat-
tery), the pump will never run out of pressure.
Temperature
Correction Chart.
030815
tools.eps2
 Loading...
Loading...
