Introduction and Safety
Page 1-2
1.1. Overview
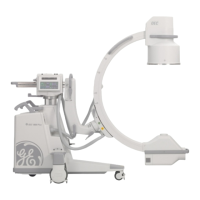
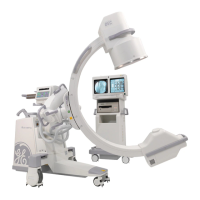
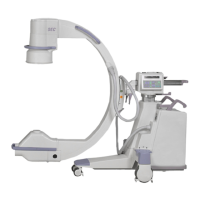
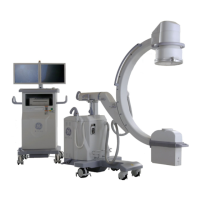
This manual describes operation for Brivo OEC 715/785/865 Mobile C-Arm X-Ray Product only. It is intended
for qualified medical personnel who have been trained in the use of medical imaging equipment. It is not
designed to replace or substitute for certified training in the radiological or medical field.
The Brivo OEC 715/785/865 Mobile C-Arm X-Ray Product is designed to provide digital spot-film imaging
and fluoroscopic image guidance across all adult and pediatric populations for orientations between
patient anatomy and surgical instruments. The product is used for general surgical applications and
musculoskeletal procedures to visualize, for example, implant localization/s or needle positions for
aspirations, injections or biopsy. Not for interventional use.
Contraindications: pregnant or lactating women, persons who can’t tolerate surgery, persons who have
mental disorders and can’t cooperate in surgery, etc.
1.2. Owner Responsibilities
The owner has the responsibility to ensure system compatibility, operator qualifications and the continued
compliance of equipment and operating specifications. The owner has the responsibility to ensure that only
properly trained, qualified personnel who have obtained credentials from the appropriate authorities
operate the system. Systems should only be used in designated use areas with approved AC receptacles.
Unauthorized changes or modifications to any part of the system could have hazardous consequences.
Changes or modifications must not be made unless specifically authorized by GE HUALUN Medical Systems
Co, Ltd.
1.2.1. System Compatibility
Damage may result to the system if incompatible components are connected. Read your operator manual
thoroughly prior to connecting components that you are uncertain it’s compatible.
1.2.2. Operator Qualifications
It is the responsibility of the owner to ensure that the system is operated only by properly trained, qualified
personnel who have obtained credentials from the appropriate authorities.
1.2.3. Continued Compliance
The owner is responsible for verifying continued compliance with all applicable regulations and standards.
Consult local, state, federal and/or international agencies regarding specific requirements and regulations
applicable to the use of this type of medical electronic equipment.
International standards include the following but not limited:
• US Federal Performance standard 21CFR 1020.30; 21CFR 1020.31; 21CFR1020.32
• IEC 60601-1 1988 (2nd Edition) +Amd. 1 & Amd. 2/EN 60601-1 (1990) + Amd. 1 & Amd. 2, Medical
electric equipment Part 1: General requirements for safety
• IEC 60601-1-1 2000 2nd Edition, Medical electrical equipment Part 1-1 - Collateral Standard: Safety
Requirements for Medical Electrical Systems
 Loading...
Loading...