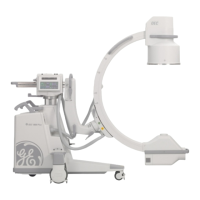
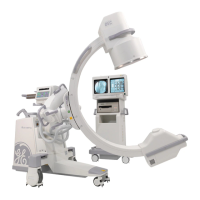
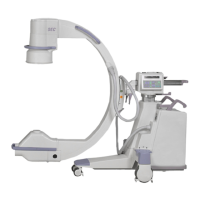
Brivo OEC 715/785/865 Mobile C-Arm X-Ray Product
Page 1-3
• IEC 60601-1-2 (2001) & Amd. 1 (2004)/EN 60601-1-2 (2001) & Amd. 1 (2006), Medical electrical
equipment Part 1-2:Collateral standard: Electromagnetic compatibility –Requirements and tests
• IEC 60601-1-3 (1994)/EN 60601-1-3 (1994), MEDICAL ELECTRICAL EQUIPMENT Part 1-3: Collateral
Standard: General Requirements For Radiation Protection In Diagnostic X-Ray Equipment
• IEC 60601-1-4 (1996) & Amd. 1 (1999)/EN 60601-1-4 (1996) & Amd. 1 (1999), Medical electrical
equipment Part 1-4 - Collateral Standard: Programmable electrical medical systems
• IEC 60601-1-6 (2006)/EN 60601-1-6 (2007), Medical electrical equipment Part 1-6: General
requirements for safety - Collateral Standard: Usability
• IEC 60601-2-7 (1998)/EN 60601-2-7 (1998), Medical Electrical Equipment Part 2: Particular
requirements for the safety of high-voltage generators of diagnostic X-Ray generator
• IEC 60601-2-28 (1993)/EN 60601-2-28 (1993), Medical electrical equipment Part 2: Particular
requirements for the safety of X-Ray source assemblies and X-Ray tube assemblies for medical
diagnosis
• IEC 60601-2-32 (1994)/EN 60601-2-32 (1994), Medical electrical equipment Part 2: Particular
requirements for the safety of associated equipment of X-Ray equipment
• IEC 60601-1 (2005)/EN 60601-1 (2006), Medical electrical equipment Part 1: General requirements for
basic safety and essential performance
• IEC 60601-1-2 (2007)/EN 60601-1-2 (2007), Medical electrical equipment Part 1-2: Collateral standard:
Electromagnetic compatibility –Requirements and tests
• IEC 60601-1-3 (2008)/EN 60601-1-3 (2008), Medical electrical equipment Part 1-3: Collateral Standard:
Radiation protection in diagnostic X-Ray equipment
• IEC 60601-1-6 (2010)/EN 60601-1-6 (2010), Medical electrical equipment Part 1-6: Collateral standard:
Usability
• IEC 60601-2-43 (2010)/EN 60601-2-43 (2010), Medical electrical equipment Part 2-43: Particular
requirements for basic safety and essential performance of X-Ray equipment for interventional
procedures
• IEC 60601-2-28 (2010)/EN 60601-2-28 (2010), Medical electrical equipment Part 2-28: Particular
requirements for the basic safety and essential performance of X-Ray tube assemblies for medical
diagnosis
1.2.4. Unauthorized Modifications
This equipment meets US Federal regulations and International standards. Unauthorized modifications to
the equipment may impact adherence to these standards and make the equipment unsafe to operate.
Never make any modifications or adjustments to the equipment unless directed by a qualified GE
Healthcare representative.
U.S. federal law restricts this device to sale by or on the order of a physician, veterinarian, or
other designated licensed practitioner as appropriate for its clinical use.
 Loading...
Loading...