Introduction and Safety
Page 1-18
To comply with the regulations applicable to an electromagnetic interface for a Group 1,
Class A Medical Device, and to minimize interference risks, the following requirements shall
be applied:
1. All cables to peripheral devices must be shielded and properly grounded. The use of
cables that are not properly shielded and grounded may result in the equipment
causing radio frequency interference in violation of the European Union Medical
Device directive and FCC regulations.
2. All of the recommended guidance regarding electromagnetic environment shall be
followed.
Guidance and manufacturer’s declaration – Electromagnetic Emissions
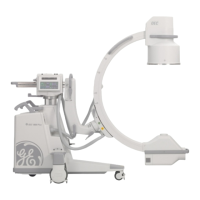
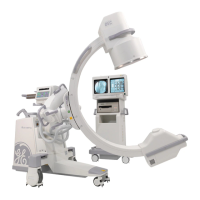
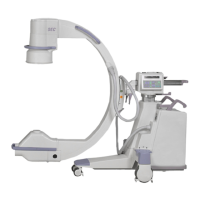
The Brivo OEC 715/785/865 Mobile C-arm X-ray Product is intended to use in the specified
electromagnetic environment. The purchaser or operator of the Brivo OEC 715/785/865 Mobile
C-arm X-ray Product should assure that it is used in an electromagnetic environment as described
below:
Electromagnetic Environment Guidance
RF Emissions
(Conducted and
radiated)
CISPR 11
The Brivo OEC 715/785/865 Mobile C-arm X-ray
Product uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not
likely to cause any interference in nearby electronic
equipment.
The Brivo OEC 715/785/865 Mobile C-arm X-ray
Product is suitable for use in all establishments other
than domestic and those directly connected to the
public low-voltage power supply network that supplies
buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
 Loading...
Loading...