Regulatory and Safety Information
RET
eval
Device User Manual 88
Sterilization
Neither the device nor the Sensor Strips require sterilization or are intended to be sterilized.
Biocompatibility
The patient-contact portion of the RET
eval
device and Sensor Strips comply with
biocompatibility standard ISO 10993-1.
Calibration and Storage
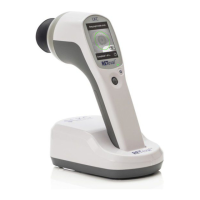
Store the device in the docking station and place dust cover over the device when
not in use.
Store the device at temperatures between -40
o
C and 35
o
C (-40
o
F and 95
o
F),
humidity between 10% and 90% non-condensing, and atmospheric pressure
between 62 kPa and 106 kPa (-4000 m to 13,000 m).
Store Sensor Strips between temperatures noted on the Sensor Strip packaging.
Short-term shipping conditions can be between -40
o
C and 70
o
C
(-40
o
F and 158
o
F), humidity between 10% and 90% non-condensing, and
atmospheric pressure between 62 kPa and 106 kPa (-4000 m to 13,000 m).
Service / Repairs
The RET
eval
device contains no user serviceable parts other than the eyecup, battery, and
electrode leads, which can all be replaced without the need of tools.
To remove the eyecup, grasp the rubber nearest the silver bezel and pull gently. To replace the
eyecup, orient the eyecup so the slots in the white plastic on the eyecup are aligned with the
bumps on the device. Push gently until the eyecup clicks into the device. Replacement eye cups
can be ordered from your local LKC representative or from LKC directly
(https://store.lkc.com/reteval-accessories).
To replace the battery, slide the battery compartment door off. Gently pull near the connector
to remove the battery. Install the new battery, and slide the battery door back into place.
To maintain proper function and compliance to regulatory requirements, do not attempt to
disassemble the device.
Other than the replacement parts mentioned above and cleaning as described elsewhere in this
manual, no user maintenance is required to maintain proper function and regulatory compliance.
Product performance
The RET
eval
device’s normal operation includes measuring flicker implicit time with a single-
patient, single-day standard deviation that is typically less than or equal to 1.0 ms; therefore, the
RET
eval
device must operate with no unintended deviations in settings and with typical
operation.
 Loading...
Loading...