1 - 8 Operator’s Manual
1 Safety Precautions
difficulty breathing (wheezing), dizziness, shock, swelling of the face, hives,
sneezing, or itching of the eyes (FDA Medical Alert on latex products, “Allergic
Reactions to Latex-containing Medical Devices”, issued on March 29, 1991).
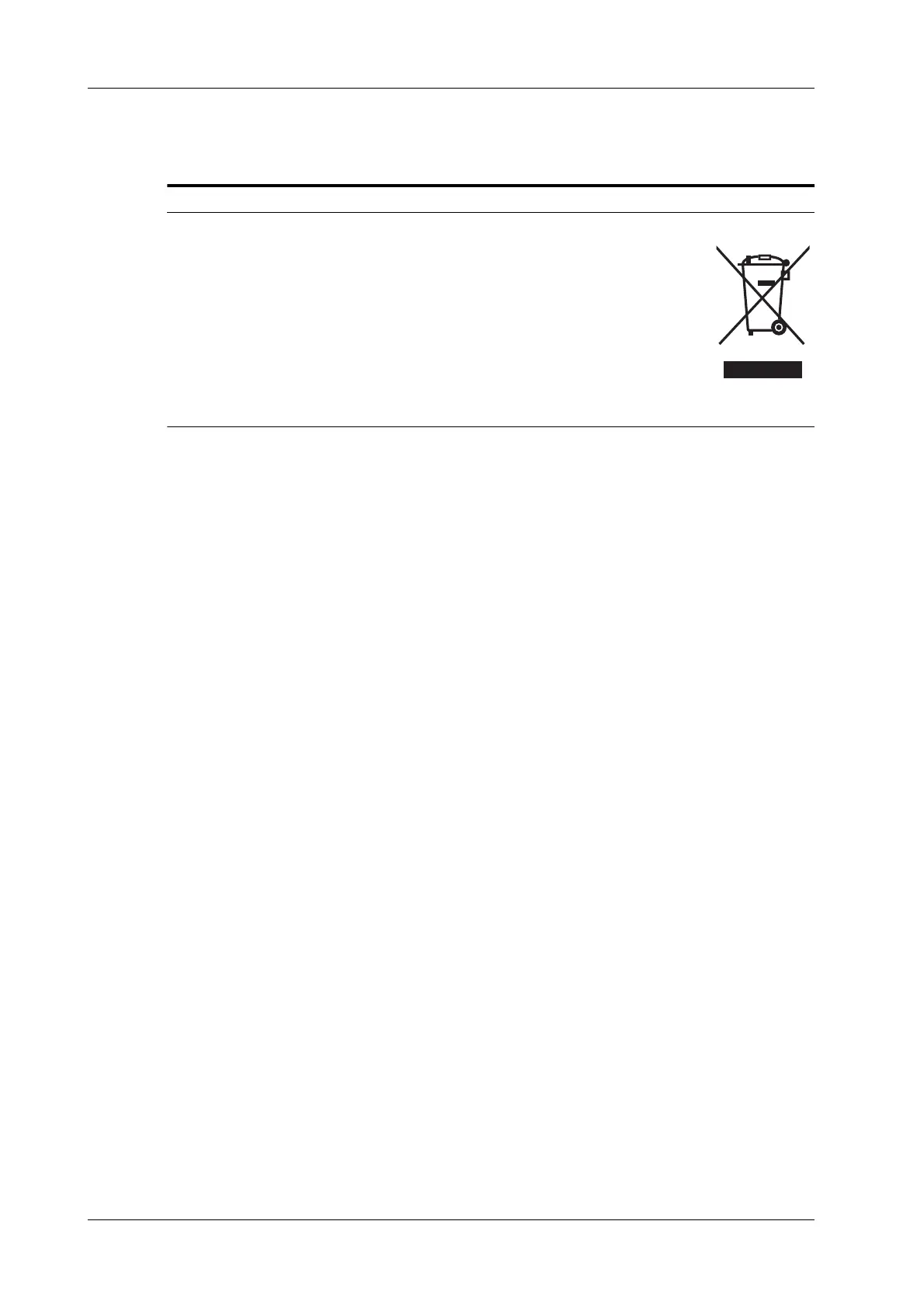
• The following definition of the WEEE label applies to EU member states
only: The use of this symbol indicates that this system should not be treated
as household waste. By ensuring that this system is disposed of correctly, you
will help prevent bringing potential negative consequences to the
environment and human health. For more detailed information with regard to
returning and recycling this system, please consult the distributor from whom
you purchased the system.
• For system products, this label may be attached to the main unit only.
 Loading...
Loading...