Preparation
Instructions for use • AIDA C WD310 • AAB272_EN_V2.0_12-2021_IFU_CE-MDR 22
5 Preparation
5.1 Unpacking the product
1. Carefully remove the product and accessories from the packaging.
2. Check the delivery for missing items and any possible damage.
3. In the case of damage, hidden defects, and short deliveries, document their nature and
extent and contact the manufacturer or supplier immediately.
4. Keep packaging for further transport.
5.2 Inspecting the device
1. Inspect the device for visible contamination. Do not use if contaminated.
2. Inspect the device for the following characteristics:
– Good working order
– Functionality
– Correct assembly of the components
– Completeness
5.3 Setting up the device
The product can be operated free-standing, in a rack, or as a wall-mounted solution in either a
vertical or horizontal position.
1. Clean and disinfect the product thoroughly before using for the first time.
2. Ensure that the ambient conditions are observed, see chapter
Ambient conditions
[p.21].
3. Ensure that the technical data is observed, see chapter
Technical data
[p.15].
4. Install the product out of reach of patients.
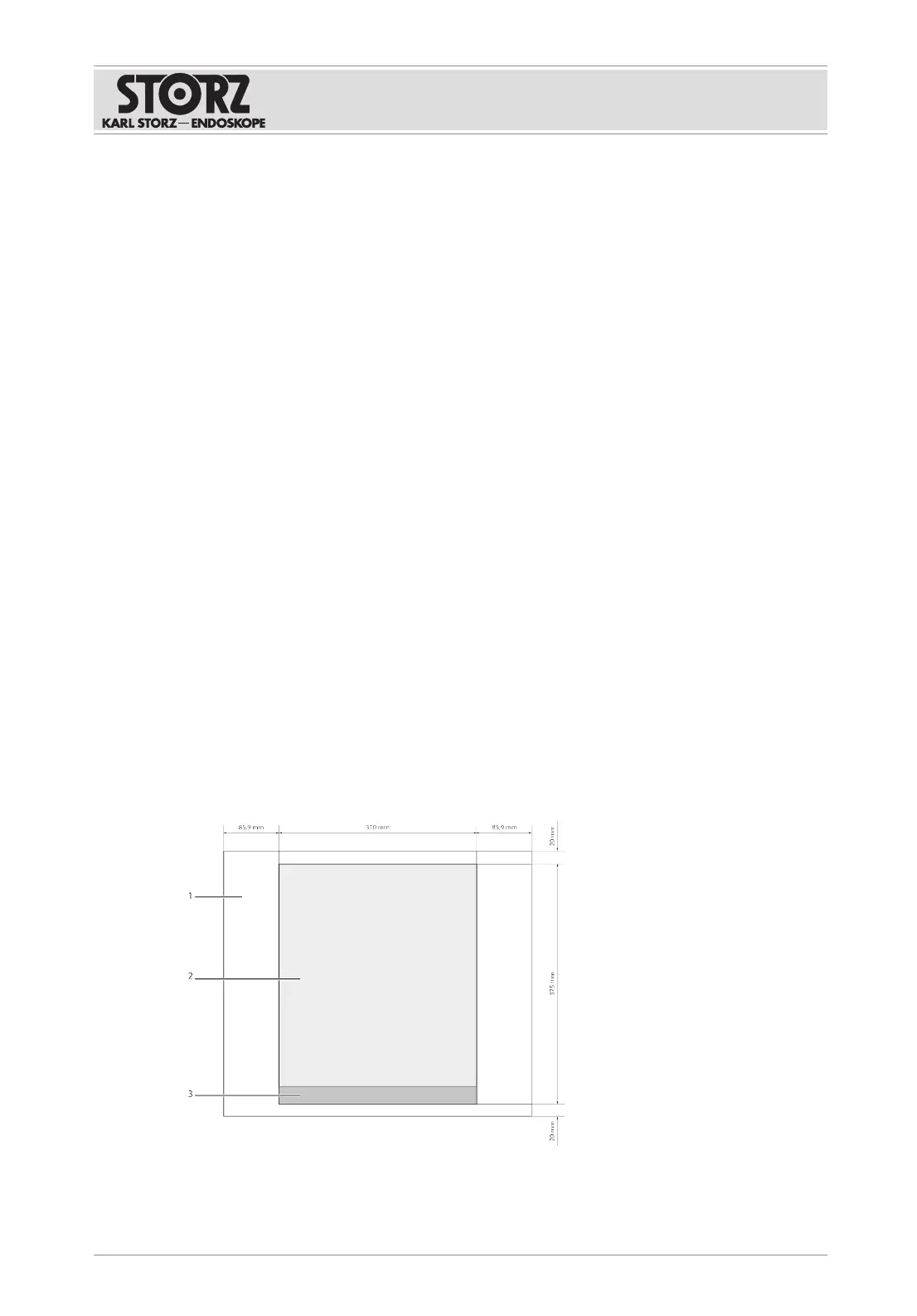
5. Note the free area dimensions based on the graphic to guarantee that the product is
sufficiently ventilated.
1 Defined free area 3 Front
2 Housing

 Loading...
Loading...











