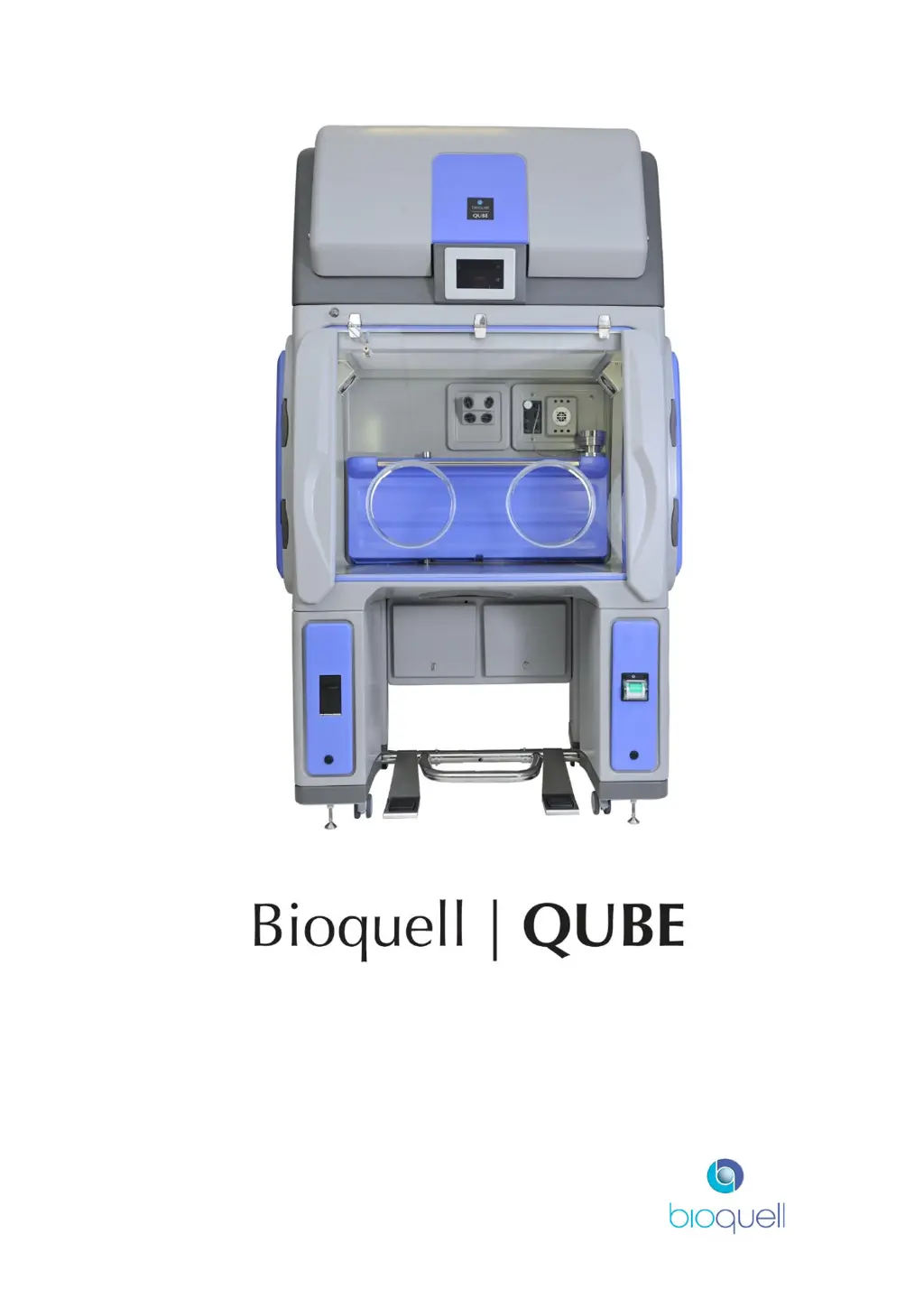
Why is the cycle time extended on my Bioquell QUBE Desktop?
- BBryan FosterJul 28, 2025
If the cycle time of your Bioquell Desktop increases, make sure that alcohols, IPA, or other hydrocarbons aren't present in the atmosphere. Avoid using them to clean the Qube or wipe items before placing them inside.