MAN-05359-001 -001 Rev. 001 page 15 of 32
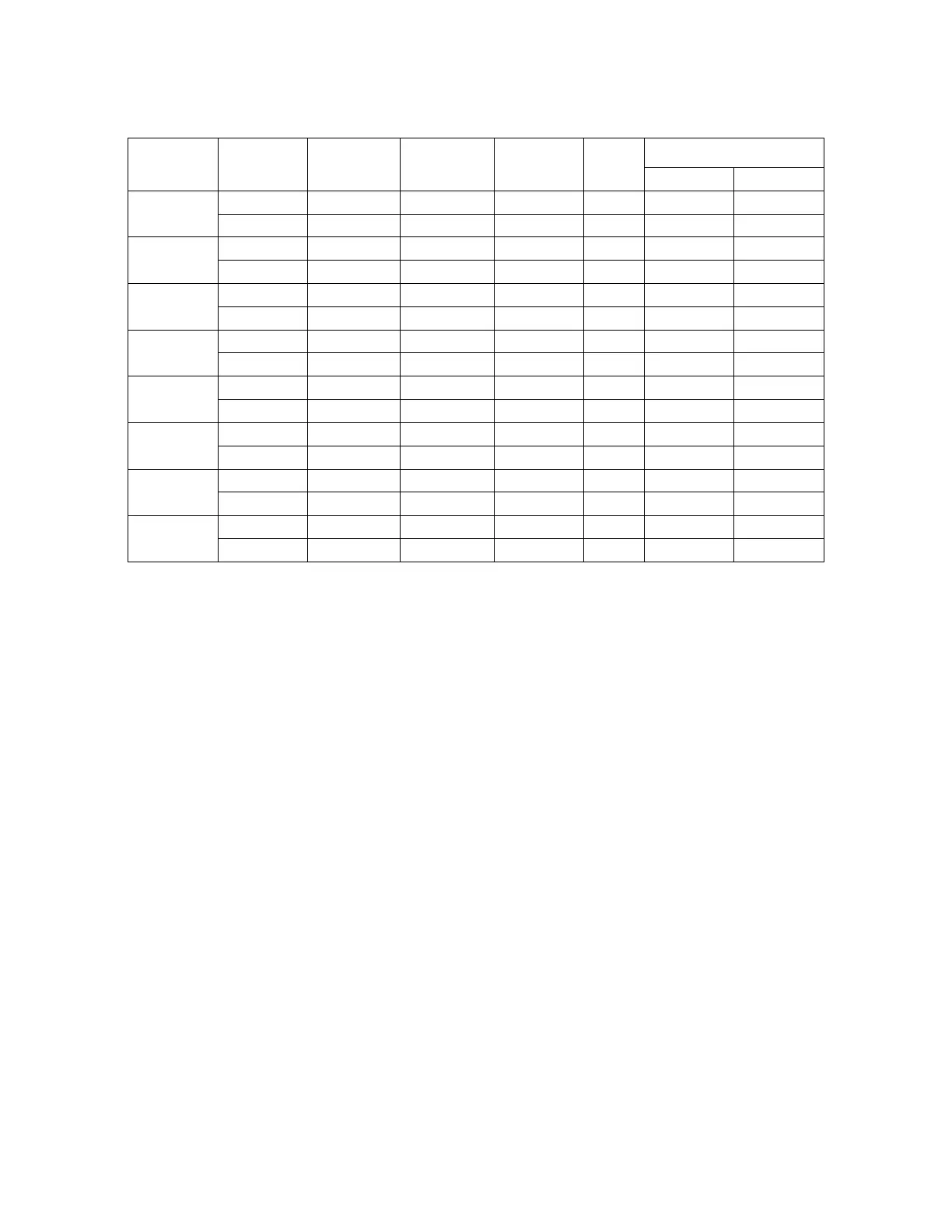
Table 16. Summarized Results of Within-instrument Study
Dx Imager
%
Abnormal
%
Category+
%
Normal
%
N/A
Abnormal FOV
% zero Median
NILM
TIS
69.6% 11.0% 70.4% 0
I2
78.1% 4.3% 78.4% 0
ASCUS
TIS
75.9% 75.9% 13.3% 25.0% 6
I2
71.9% 71.9% 5.0% 28.1% 7
LSIL
TIS
97.3% 93.2% 3.3% 2.8% 14
I2
96.0% 94.0% 0.7% 4.0% 15
ASC-H
TIS
93.3% 86.7% 0.0% 6.7% 11.5
I2
100% 83.3% 0.0% 0.0% 14
AGUS
TIS
63.0% 51.9% 6.7% 35.7% 2
I2
55.6% 48.1% 10.0% 44.4% 2
HSIL
TIS
98.0% 77.3% 0.0% 2.0% 20
I2
97.3% 71.3% 0.7% 2.7% 20
CANCER
TIS
100% 46.7% 0.0% 0.0% 22
I2
100% 53.3% 0.0% 0.0% 22
UNSAT
TIS
72.2% 40.0% 72.2% 0
I2
85.7% 36.7% 94.7% 0
Between-instrument Reproducibility
Between-instrument reproducibility results were derived from the clinical study. In the clinical
study, three (3) cytotechnologist/pathologist pairs reviewed slides on different instruments.
In Table 17, the between-instrument results are summarized for each diagnostic category of
slides (according to adjudicated truth results). For each grouping, the following metrics are
reported:
% Abnormal
The proportion of slides for which any abnormal diagnosis was recorded.
(For NILM or UNSAT slides, the % Normal column is used to record the proportion that are
not abnormal).
% Category+
The proportion of slides for which the site diagnosis was equal to or higher than the slide’s
adjudicated category.
 Loading...
Loading...