MAN-05359-001 -001 Rev. 001 page 16 of 32
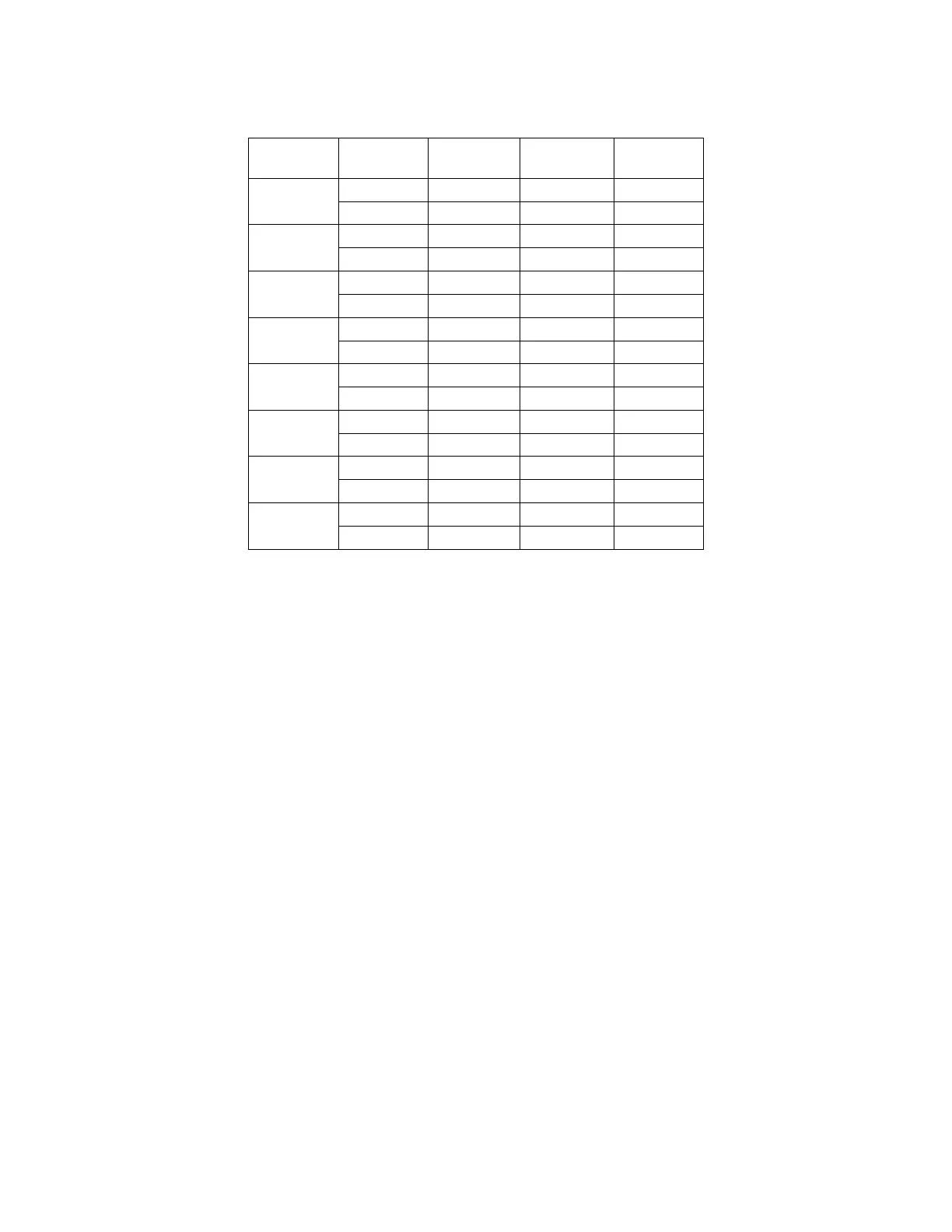
Table 17. Summarized Results of Between-instrument Study
Dx Imager
%
Abnormal
%
Category+
%
Normal
NILM
TIS
-- -- 90.0%
I2
-- -- 88.1%
ASCUS
TIS
64.4% 64.4% --
I2
71.7% 71.7% --
LSIL
TIS
95.0% 75.0% --
I2
96.9% 80.6% --
ASC-H
TIS
87.7% 62.6% --
I2
92.8% 63.6% --
AGUS
TIS
53.8% 37.6% --
I2
67.5% 57.3% --
HSIL
TIS
97.7% 54.7% --
I2
99.3% 64.7% --
CANCER
TIS
100% 63.2% --
I2
100% 63.2% --
UNSAT
TIS
-- -- 95.2%
I2
-- -- 93.2%
G2.4 Cytotechnologist Screening Rates During Clinical Study
During the study, nine (9) cytotechnologists (CTs) recorded the number of hours they worked
each day and the number of slides screened for both the TIS and I2 reviews. The experience
levels of the cytologists ranged from 4 to 30 years. During the study, the cytotechnologist’s
screening times for both TIS Review and I2 Review included automated screening of the 22
fields of view, full slide review if the automated screening was not applicable, and automated
screening of the 22 fields of view followed by full slide review when abnormal cells were
identified during automated screening. The number of hours each cytotechnologist screened
slides per day varied due to logistical issues and scheduling. Only the sequential modality of I2
Review was evaluated during clinical study.
These data are summarized in Table 18 below.
Note: These numbers represent total number of slides and does not consider the review type;
Field of view (FOV) only, Full Manual Review (FMR), or FOV+FMR. These rates are
lower than would be routinely observed in clinical practice as the number of abnormal
cases in this clinical study was much higher than typically observed in normal clinical
practice (50% versus 10–20%).
 Loading...
Loading...