Choosing a protocol
RET
eval
Device User Manual 21
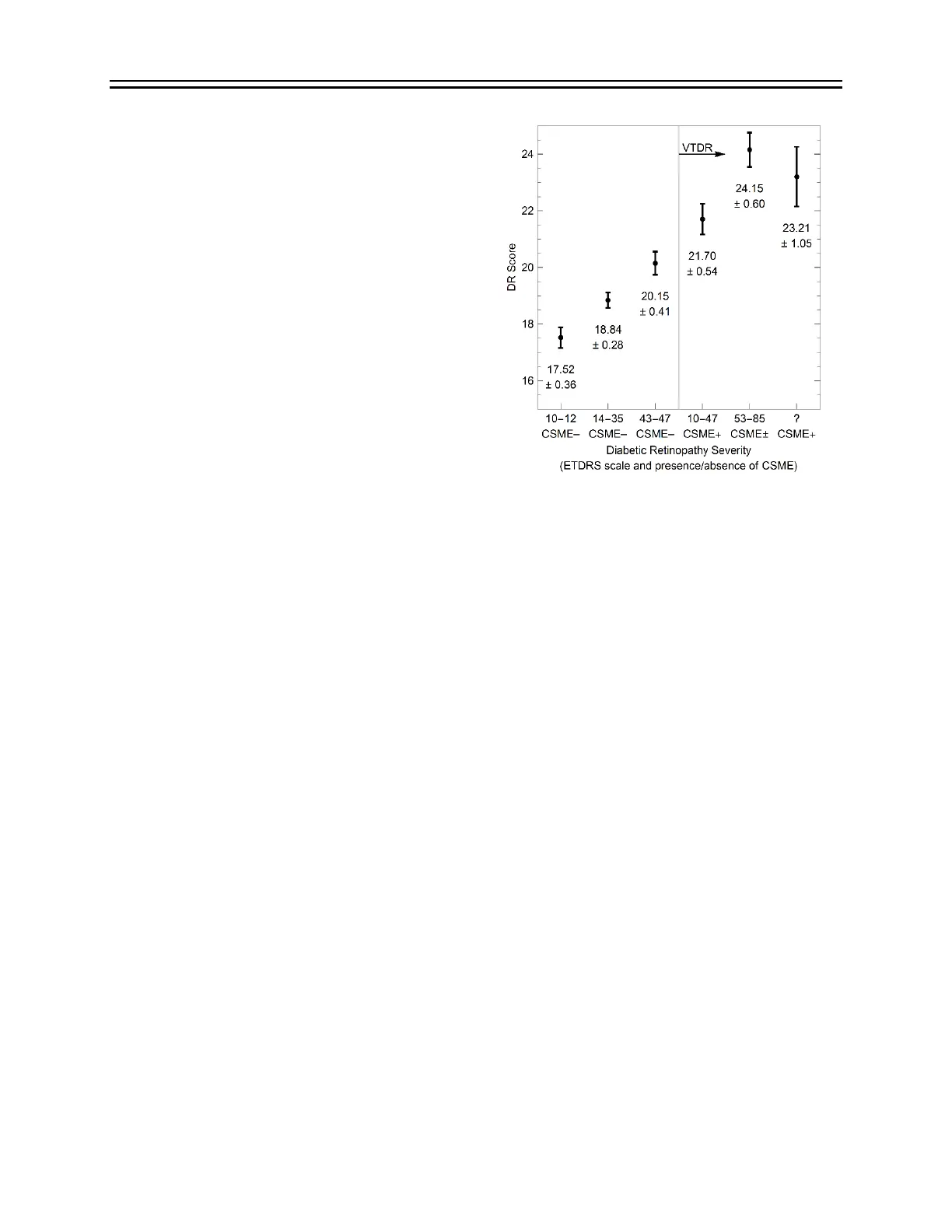
The score produced by the DR Assessment
Protocol correlates with the presence and
severity of diabetic retinopathy and clinically
significant macular edema, as shown in Figure 1
(Maa et al. 2016).
Figure 1. Dependence of RETeval measurements on diabetic
retinopathy severity level. Plots show the mean and standard
error of the mean for each severity group listed in Table 1.
The DR Assessment Protocol uses two or three
sets of 4, 16, and 32 Td∙s flickering white stimuli
(28.3 Hz) with no background light. The number
of sets is determined by the device’s internal
precision metrics. The Troland unit (Td) describes
retinal illuminance, which is the amount of
luminance that enters the pupil. The RET
eval
device measures the pupil size in real time and continuously adjusts the flash luminance to deliver
the desired amount of light into the eye regardless of the size of the pupil. The light stimuli are
white light (1931 CIE x, y of 0.33, 0.33).
The patient’s result is a combination of the following:
• Age of the patient
• The timing of the electrical response to the 32 Td∙s stimulus
• The amplitude of the electrical response to the 16 Td∙s stimulus
• The ratio of the pupil area between the 4 Td∙s stimulus and the 32 Td∙s stimulus
To help ensure accurate results, enter the correct birthdate.
Individuals with diabetes who have severe retinopathy typically have pupils that change size less
than the pupils of healthy individuals. If the patient is on medications or has other conditions that
impair the pupil response, extra care must be taken to properly interpret the RET
eval
device
results, as these individuals are more likely to be erroneously classified as likely to have vision
threatening DR. Further, ensure the contralateral eye is covered by the patient’s hand, as shown
on Page 13 to prevent uncontrolled light stimulation of the contralateral eye from affecting the
pupil being measured. Do not use the DR Assessment Protocol on patients whose eyes are
pharmacologically dilated.
The report generated by the DR Assessment Protocol includes reference intervals for each
individual measurement and the DR Score, from our studies of normally-sighted subjects. See
the Reference Intervals section in the manual (starting on page 67) for further details. These
reference intervals enable you to compare the results to a cohort of subjects who do not have
diabetes nor diabetic retinopathy, and also to more easily identify which aspects of a test are
more concerning.
 Loading...
Loading...