94
GB
8 Dismantling / Mounting in Case of Service
1505093, Edition 2012-05, Version 5
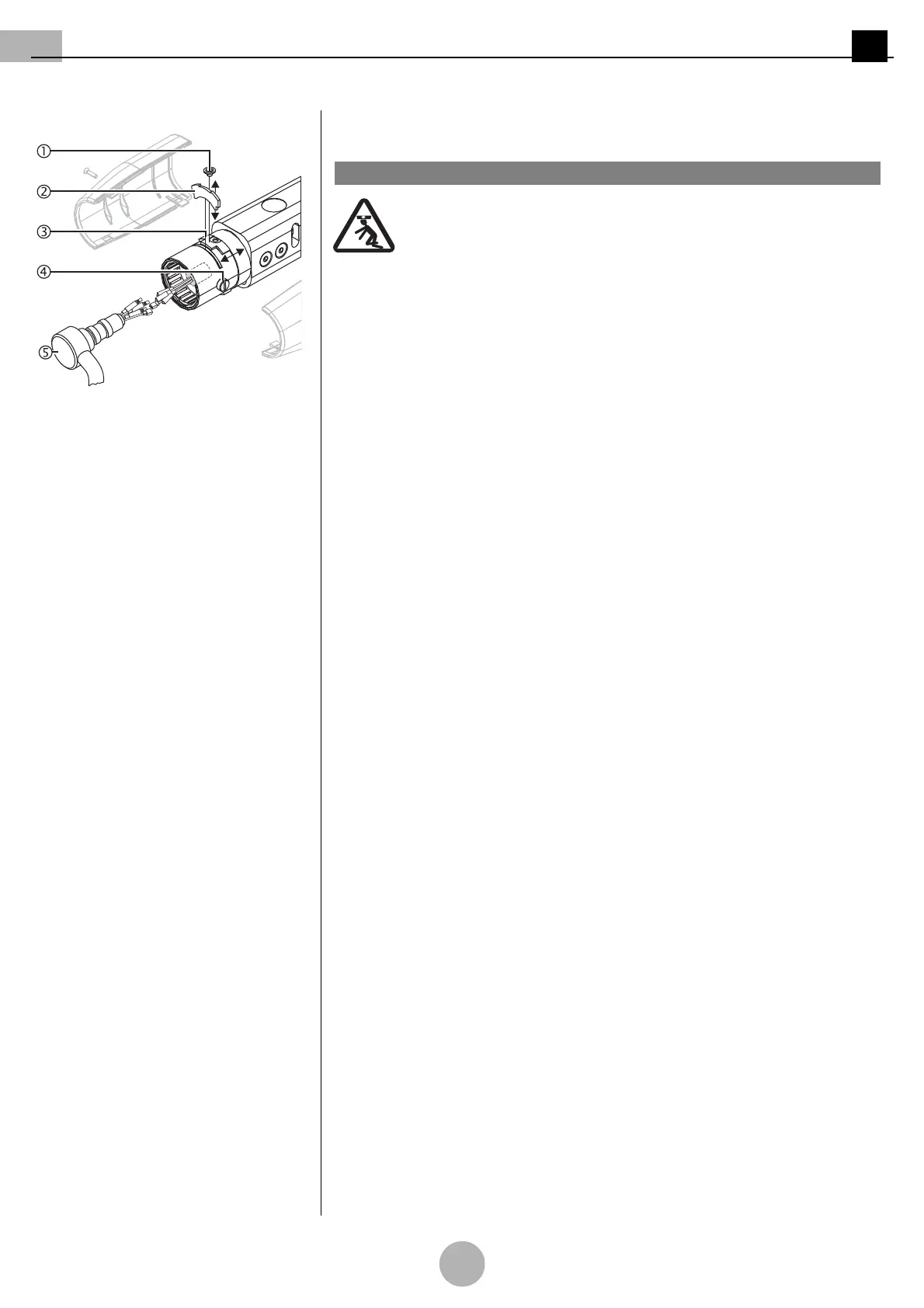
Figure 21: Completing the installation Completing the installation
(See "Figure 21")
End device crashing
When inserting an incorrect securing segment 2, the adaptation / end
device can crash and the LCH spring arm 6 can suddenly jump up:
• Only use a genuine securing segment 2.
The securing ring 3 secures the securing segment 2 and thus ensures
that the end device is securely in place in the LCH spring arm 6:
•The securing ring 3 must cover the securing segment 2.
•The Allen countersunk screw M4 x 4mm 1 must be screwed in and
tightened.
1. Insert a genuine securing segment 2.
2. Push the securing ring for the securing segment 3 forwards and then screw in and
tighten the Allen countersunk screw M4 x 4 mm 1 through the securing ring for the
securing segment 3.
3. Check that the pivot 6 of the end device (e.g. flat screen, OR lamp, etc.) is securely
in place.
•The pivot 6 must sit tightly in the spring arm.
•The pivot 6 must rotate easily without resistance.
4. Slightly tighten the opposite brake screws 4.
Extending the vertical lift
5. If required, extend the vertical lift of the LCH spring arm 6 as described in
“Chapter
9” on page 95
.
Adjustments
6. Adjust the brake force and spring tension as described in
“Chapter 9” on page 95
.
Mounting the cover panels
7. Mount the cover panels as described in
“Chapter 8.7” on page 91
.
Function test
8. Connect the system to the mains.
9. Perform a function test.
ACROBAT 2000 appliances are only intended for the connection of luminaires for medical
diagnosis, surgical luminaires and flat screens bearing the CE mark and with a maximum
payload of 21 kg.
For technical assistance for creating a medical-electrical (ME) system refer to
DIN EN 60601-1, Part 16.
The manufacturer / marketer of the ME system must provide a declaration in accordance
with Article 12 of 93/42/EEC (Medical Device Directive, MDD).
1WARNING
 Loading...
Loading...