Page 49 / 53
Pulse modulation 217Hz, 28V/m
Pulse modulation 217Hz, 28V/m
Pulse modulation 217Hz, 9V/m
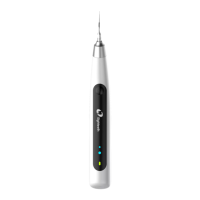
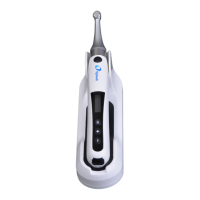
13.3 EMC Standards for the HELIOS 600
IEC 60601-1-2: 2014 EMC requirements and tests, Medical Electrical Equipment
including CSIPR11:2009+A1:2010 Group 1, Class B.
13.4 Accessories
The use of cables or accessories other than those specified, with the exception of those
sold by the manufacturer of the equipment, as replacement parts for internal
components may result in increased emissions or decreased immunity of the medical
equipment.
13.5 Other Equipment
Use of this equipment adjacent to or stacked with other equipment
should be avoided because it could result in improper operation. If such use is
necessary, this equipment and the other equipment should be observed to verify
normal operation.
13.6 Regulatory Information
The HELIOS 600 complies with the following regulations:
(EU) 2017/745 Medical Device Regulation (MDR), Cass I following the Rule 5.
FDA Center for Devices & Radiological Health CDRH - Title 21 CFR 872.3661
(USA). Medical Devices Regulations (Canada).
Directive 2011/65/EU on the restriction of the use of certain hazardous
substances in electrical and electronic equipment (RoHS).
 Loading...
Loading...