6S2
0RQLWRULQJ
Patient Monitor Using SpO2
9-5
Figure 9-2
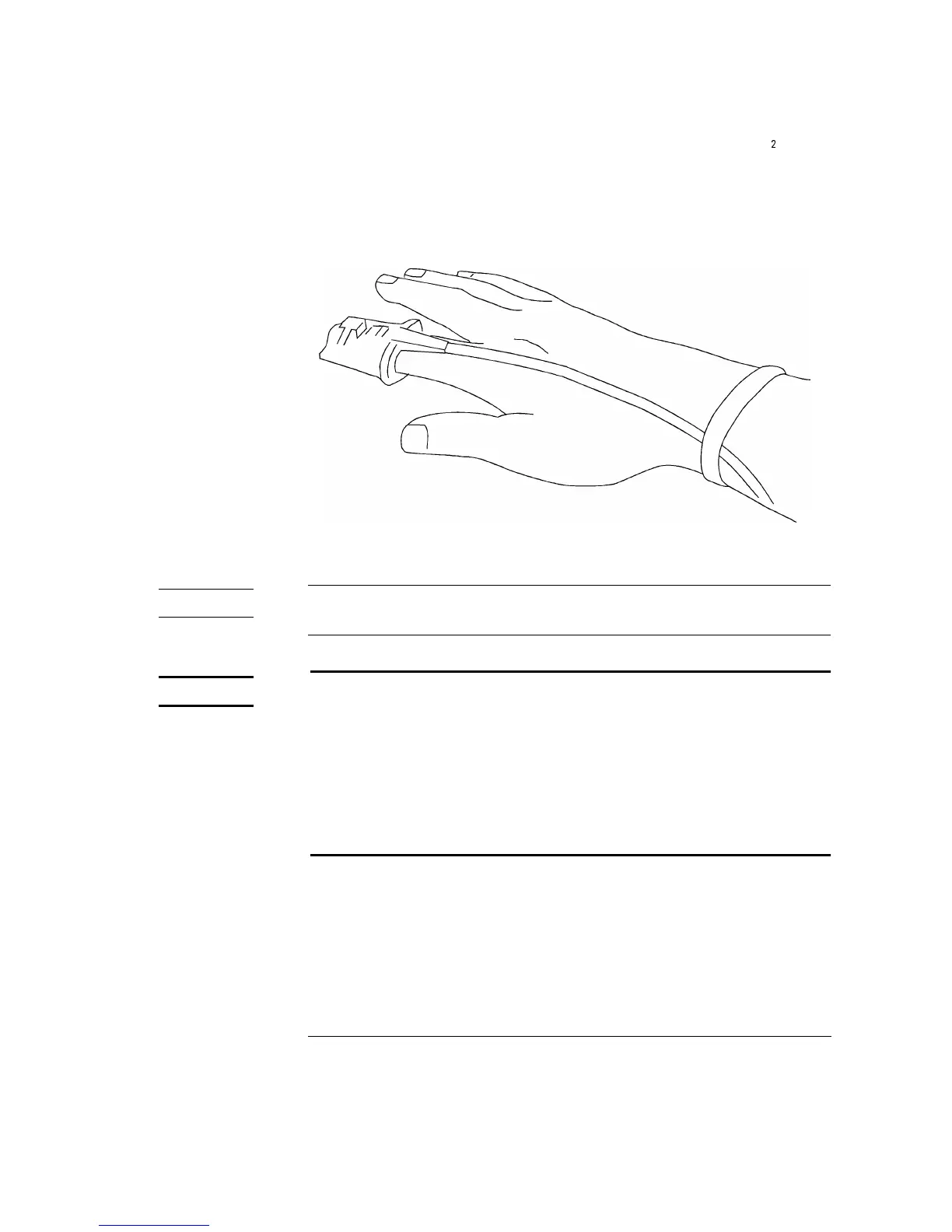
Applying the HP M1190A Reusable Sensor
9-2
When non-HP SpO
2
sensors are used, application must be consistent with the
manufacturer’s own guidelines.
Prolonged, continuous monitoring may increase the risk of undesirable
changes in skin characteristics, such as irritation, reddening, blistering or
pressure necrosis, particularly on neonates and on patients with impaired
perfusion and varying or immature skin morphology. Specific attention
must be given to sensor site inspection for changes in skin quality, proper
optical path alignment and attachment. Check the application site at
regular intervals — at least every two hours — and change the site if any
compromise in skin quality should occur. More frequent checking may be
required due to an individual patient's condition.
 Loading...
Loading...