2-7
Questions? Refer to Page 11-22 For Technical Support Instructions.
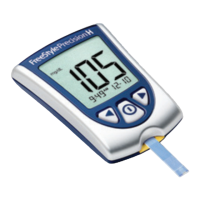
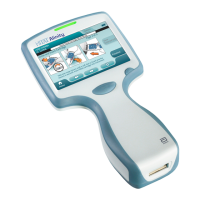
Chapter 2: Using the Meter
e 802.11a/b/g wireless radio technology in this product is in compliance with the Class B Information Technology Equipment
requirements and other relevant provisions of European Union Radio and Telecommunications Terminal Equipment Directive
1999/5/EC. e limits for Class B equipment were derived for typical residential environments to provide reasonable protection
against interference with licensed communications devices. e internal function is a radio device using the 2.4 GHz frequency
band (2.400GHz - 2.4845 GHz) and 5 GHz frequency band (5.150-5.250 GHz, 5.725-5.825 GHz). It is intended for wireless
communication with other 802.11a/b/g-enabled devices in an indoor environment.
e use of 802.11a/b/g wireless technology in certain countries may be restricted. Before using 802.11x products, please conrm
with the frequency management authority in the country where you plan to use it. Some countries allow indoor use only. In
some situations or environments, the use of 802.11x wireless technology might be restricted by the proprietor of the building or
responsible representatives of the organisation, for example, in aeroplanes, in hospitals or in any other environment where the
risk of interference with other devices or services is perceived or identied as harmful.
If you are uncertain of the policy that applies to the use in a specic organisation or environment, you are encouraged to ask
for authorisation to use 802.11x wireless technology prior to switching it on. Consult your physician or the manufacturer of
personal medical devices (pacemakers, hearing aids, etc.) regarding any restrictions on the use of 802.11x wireless technology.
Changes or modications made in this device, which are not expressly approved by Abbott Diabetes Care Inc., could void the
user’s authority to operate the equipment.
 Loading...
Loading...