1-1
Questions? Refer to Page 11-22 For Technical Support Instructions.
Overview
Intended Use
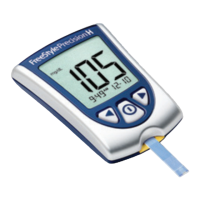
e FreeStyle Precision Pro Blood Glucose and β-Ketone Monitoring System is intended for in vitro (outside the body) diagnostic
use for the quantitative measurement of glucose (D-glucose) in fresh capillary whole blood (ngertip), and of ketone (beta-
hydroxybutyrate) in fresh capillary whole blood samples. e FreeStyle Precision Pro system is for professional use. e system
is not for use in diagnosing diabetes mellitus, but is to be used as an aid in monitoring the eectiveness of diabetes control
programmes.
Healthcare professionals may also use the product for the quantitative measurement of glucose in venous, arterial or neonatal
whole blood and ketone in venous blood, provided the sample is used within 30 minutes aer collection.
e FreeStyle Precision Pro system simplies point-of-care testing for healthcare professionals, providing features that enhance
the reliability of the testing process and that support compliance with point-of-care policies.
Important Safety Instructions
DANGER
• Misuse of electrical equipment can cause electrocution, burns, fire and other hazards.
• Basic safety precautions should always be taken, including all those listed below.
• Close supervision is necessary when equipment is used by, on, or near children, or people with disabilities.
Chapter 1: Overview

 Loading...
Loading...