INTRODUCTION
1
Art: 714363-00U Rev. Date: 02-Aug-12 1-1
This Manual
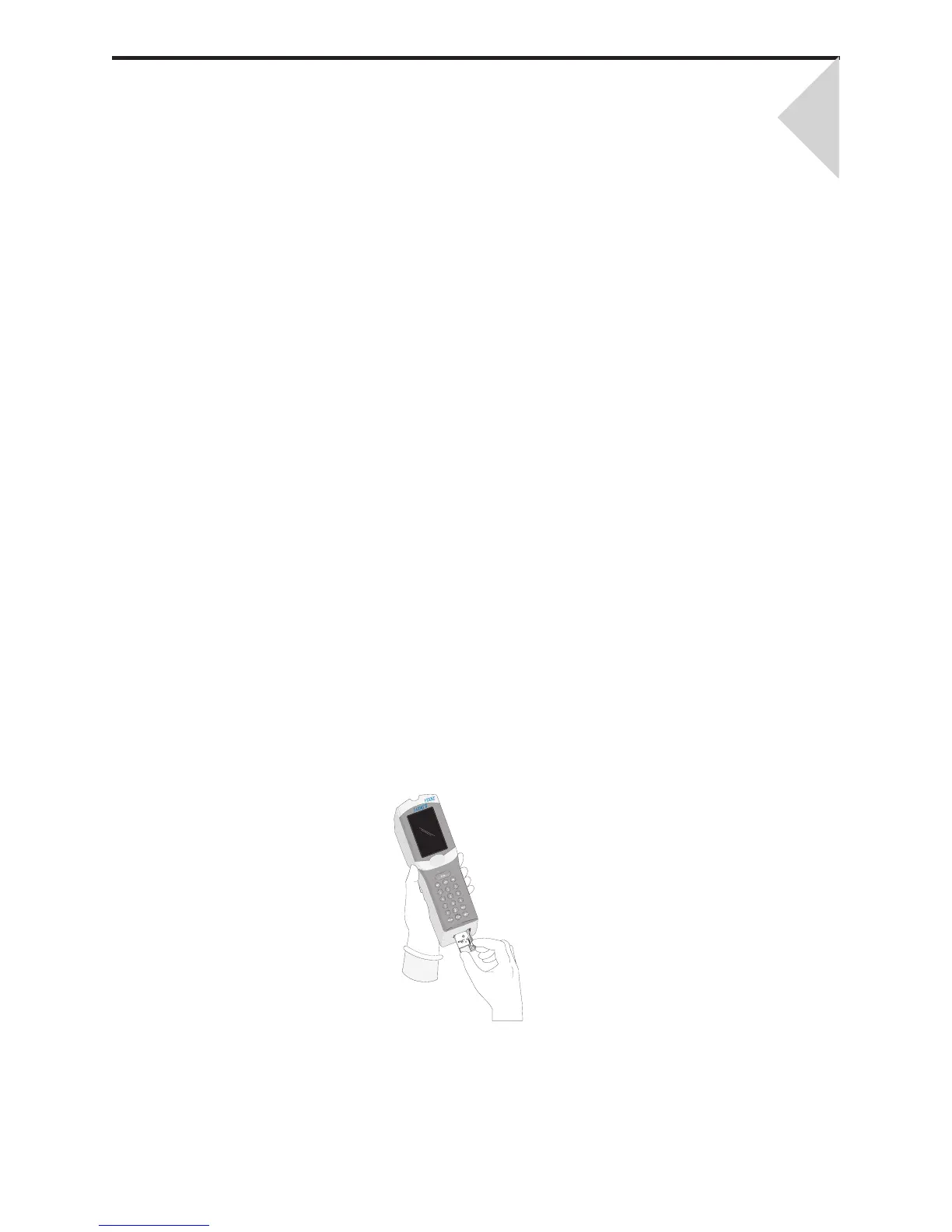
This manual describes the i-STAT
1 Analyzer and the Central Data Station software.
Related sections are grouped behind tabs.
Intended Use
The i-STAT 1 Analyzer is intended for use with i-STAT cartridges for the in vitro
quantification of various analytes in whole blood. Analyzers and cartridges should
be used by healthcare professionals trained and certified to use the system and
should be used according to the facility’s policies and procedures.
Overview of the
i-STAT System
The i-STAT System incorporates a comprehensive group of components needed
to perform blood analysis at the point of care. A portable handheld analyzer, a
cartridge with the required tests, and 2-3 drops of blood will allow the caregiver
to view quantitative test results for blood gas, chemistry and coagulation tests in
approximately two minutes.
Portable printers and infrared communication devices allow all patient information
obtained at the bedside to be printed on demand and transmitted to centralized
information systems for record keeping and billing.
The Central Data Station program provides system management tools including
real-time monitoring of testing and operator competency.
FDA Test
Categorization
With the i-STAT 1 System, the FDA has categorized the tests included on the
i-STAT G, Crea, E3+, EC4+, 6+, and CHEM8+ cartridges as waived when testing
is performed using venous whole blood samples collected in sodium or lithium
heparin evacuated tubes. Other venous whole blood samples, capillary and/or
arterial samples tested using these same cartridges on the i-STAT 1 System are
categorized by the FDA as moderate complexity.
For waived testing, laboratories are required to follow the manufacturer’s
requirements for the testing. They may elect to perform additional quality control
testing (such as the QC required for a moderate complexity test) but this does not
change the FDA categorization of the test as waived or release the laboratory’s
responsibility to follow the manufacturer’s instructions for it as a waived test.
Other testing performed with the i-STAT 1 System (other than the testing
performed using the aforementioned cartridges) is FDA categorized as "moderate
complexity".

 Loading...
Loading...