Abbott Point of Care Inc. • Abbott Park, IL 60064 • USA
Art: 721296-00A Rev. Date: 07/16/08
Technical BulleTin
i-STAT
®
THE PRESENCE OF LATEX RUBBER IN i-STAT
SYSTEM COMPONENTS
Introduction
This Technical Bulletin provides information on the presence of latex rubber in the components of the i-STAT
1 Analyzer, i-STAT Portable Clinical Analyzer and i-STAT Cartridges.
i-STAT 1 Analyzer
The exterior parts of the i-STAT 1 analyzer are latex free. No natural or synthetic latex is used anywhere on
the exterior of this product, the product packaging, or accessories.
i-STAT Portable Clinical Analyzer
The exterior parts of the i-STAT Portable Clinical Analyzer are latex free. No natural or synthetic latex is used
anywhere on the exterior of this product, the product packaging, or accessories.
i-STAT Cartridges
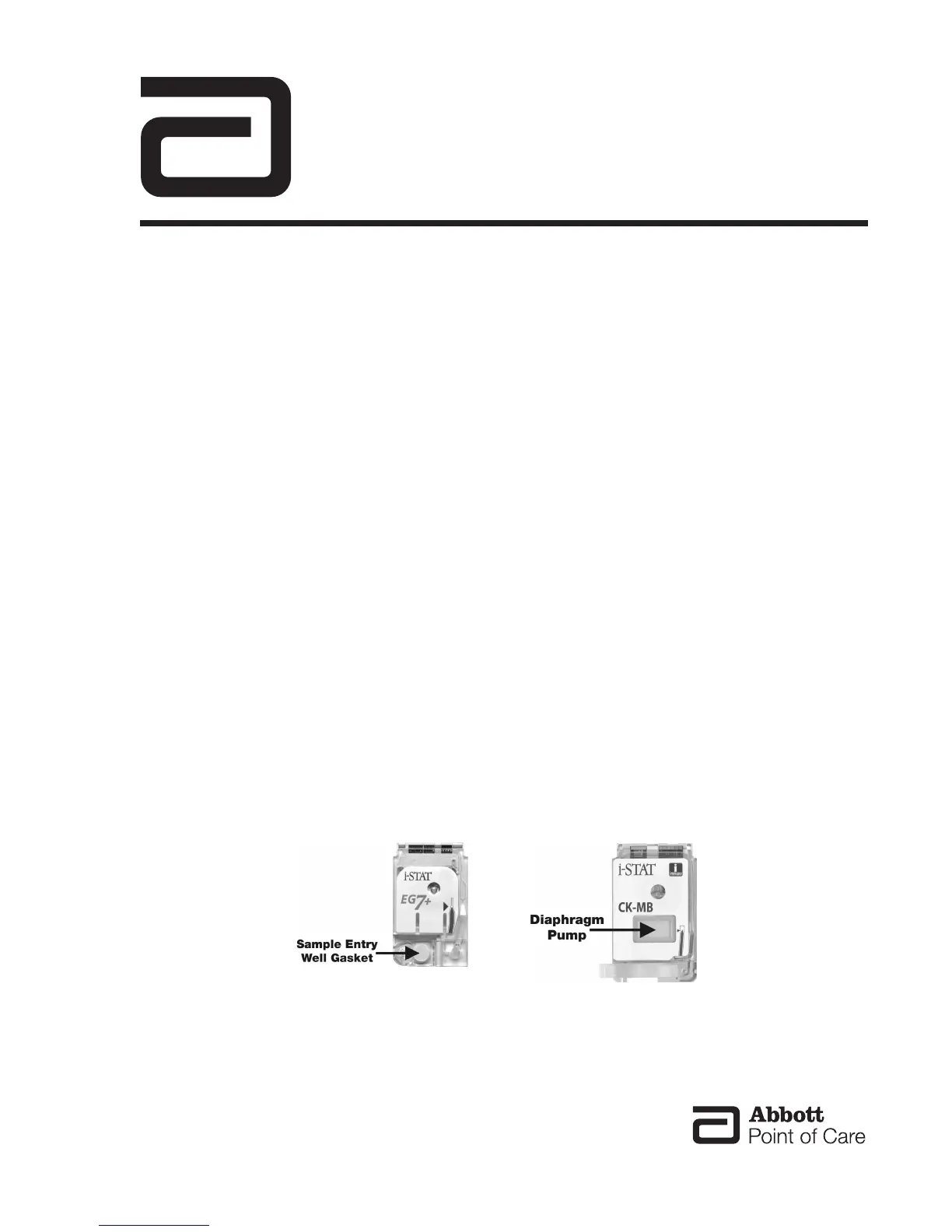
The i-STAT cartridges contain natural latex in the areas indicated below.
The ‘Sample Entry Well Gasket’ contains natural latex and is used on the following cartridges: EC8+, CG8+,
EG7+, CHEM8+, EG6+, CG4+, 6+, G3+, EC4+, E3+, G, Crea, ACTk, ACTc, and PT/INR. The location of the
‘Sample Entry Well Gasket’ is shown below.
A diaphragm pump on the following cartridges contains natural latex rubber: cTnI, CK-MB, and BNP. The
location of the diaphragm pump is shown below.
i-STAT is a registered trademark of Abbott Laboratories.
 Loading...
Loading...