Rev. Date: 12-Jul-11 Art: 714176-00L BUN - 3
Method Comparison (mg/dL)
Beckman Coulter
LX20
Dade Dimension
RxL-Xpand
Beckman Coulter
CX9
n 39 32 26
Sxx 0.36 0.48 0.39
Syy 0.67 0.34 0.60
Slope 1.03 1.05 1.00
Int’t 1.39 -0.28 -0.38
Sy.x 0.99 0.31 0.85
Xmin 5 5 7
Xmax 70 38 66
r 0.997 0.998 0.997
Cartridge Comparison
The performance characteristics of the sensors are equivalent in all cartridge configurations. System
difference analysis was performed on 40 patient samples using the i-STAT 6+ and i-STAT EC8+ cartridges.
In the 25–60 mg/dL range the average difference was -1.13. In the 60–140 mg/dL range the average
difference was -0.77.
Factors Affecting Results*
Endogenous ammonium ions will not affect results.
Test concentrations used were as per the CLSI guidance document,
6
unless otherwise indicated.
The following substances were shown not to significantly interfere with the i-STAT BUN assay at
the stated test concentrations:
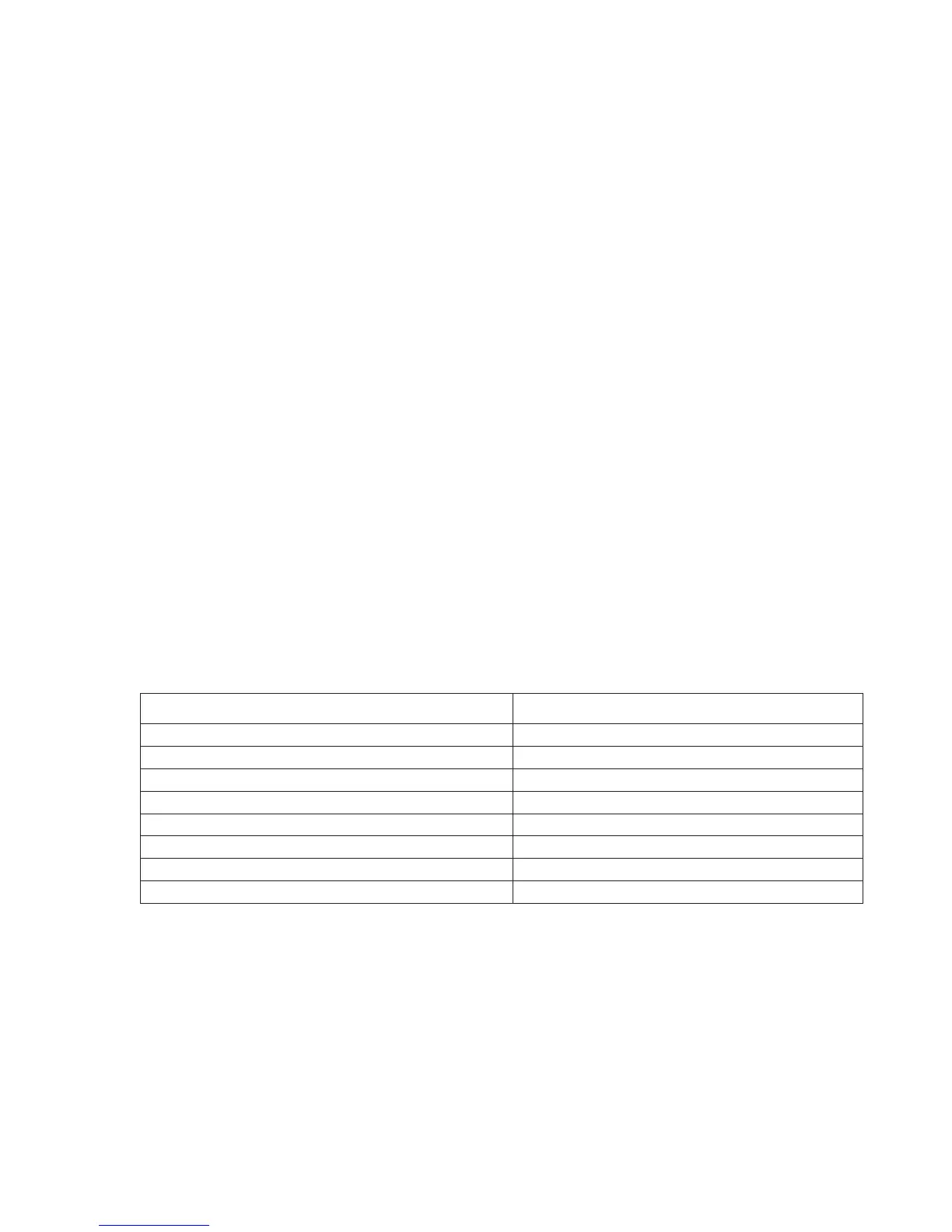
Substance
Test Concentration
(mmol/L)
Acetaminophen 1.32
Acetylcysteine 10.2
Ascorbate 0.34
Bromide 37.5
β-Hydroxybuterate 6.0
7
Lactate 6.6
Salicylate 4.34
Thiocyanate 6.9
*It is possible that other interfering substances may be encountered. The degree of interference at concentrations other than those listed might not
be predictable.

 Loading...
Loading...