2 Warnings cautions and notices 6
fabian Therapy evolution | SW V5.1.x
Ref: 121003.EN / Date: 26Jan2021
WARNING
: This VENTILATOR must NOT be used with nitric oxide.
Before use, carefully read the following literature:
• Oximetry Sensor Directions for Use
• PC-Series Patient Cable Directions for Use
EXPLOSION HAZARD: DO NOT use the oximeter in the presence of
flammable anesthetics or other flammable substances in combination with
air, oxygen-enriched environments, or nitrous oxide.
Check alarm limit settings each time the oximeter is used.
A pulse oximeter should NOT be used as an Apnea monitor.
A pulse oximeter should be considered an early warning device. As a trend
towards patient deoxygenation is indicated, blood samples should be
analyzed by a laboratory co-oximeter to completely understand the patient's
condition.
Always remove the sensor from the patient and completely disconnect the
patient from the oximeter before bathing the patient.
DO NOT use malfunctioning equipment. Have the unit repaired by Masimo or
a qualified service person.
ELECTRIC SHOCK HAZARD: DO NOT remove the oximeter cover. There are
no user-serviceable items inside the oximeter.
• An operator may only perform maintenance procedures specifically
described in this manual.
• ONLY connect IEC 60601-1 or IEC 60950-1 compliant devices to Ethernet,
Nurse call, and RS232 ports.
If the accuracy of any measurement by the oximeter does NOT seem
reasonable, first check the patient's vital signs by alternate means, and then
check the oximeter for proper functioning.
WARNING
: DO NOT use the ventilator in association with HF (High Frequency)
electrosurgical equipment.
Maintenance
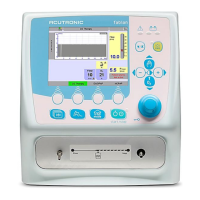
The fabian Therapy evolution device is a ventilator classified as Class IIb (for prolonged use more
than 24 hours and less than 30 days) according to the European Medical Device Directive, as
such:
• Inspection as per manufacturer specifications is required every 12 months.
• Maintenance must be performed by ACUTRONIC Medical Systems trained experts with access
to appropriate test and measuring equipment.
ACUTRONIC Medical Systems AG representatives are strongly recommended for service
agreements and repairs.
Only use original ACUTRONIC Medical Systems parts for repairs.
Note chapter “14: Ventilator service and maintenance intervals”
 Loading...
Loading...