Foreword
13
OsmoTECH® Single-Sample Micro-Osmometer User Guide
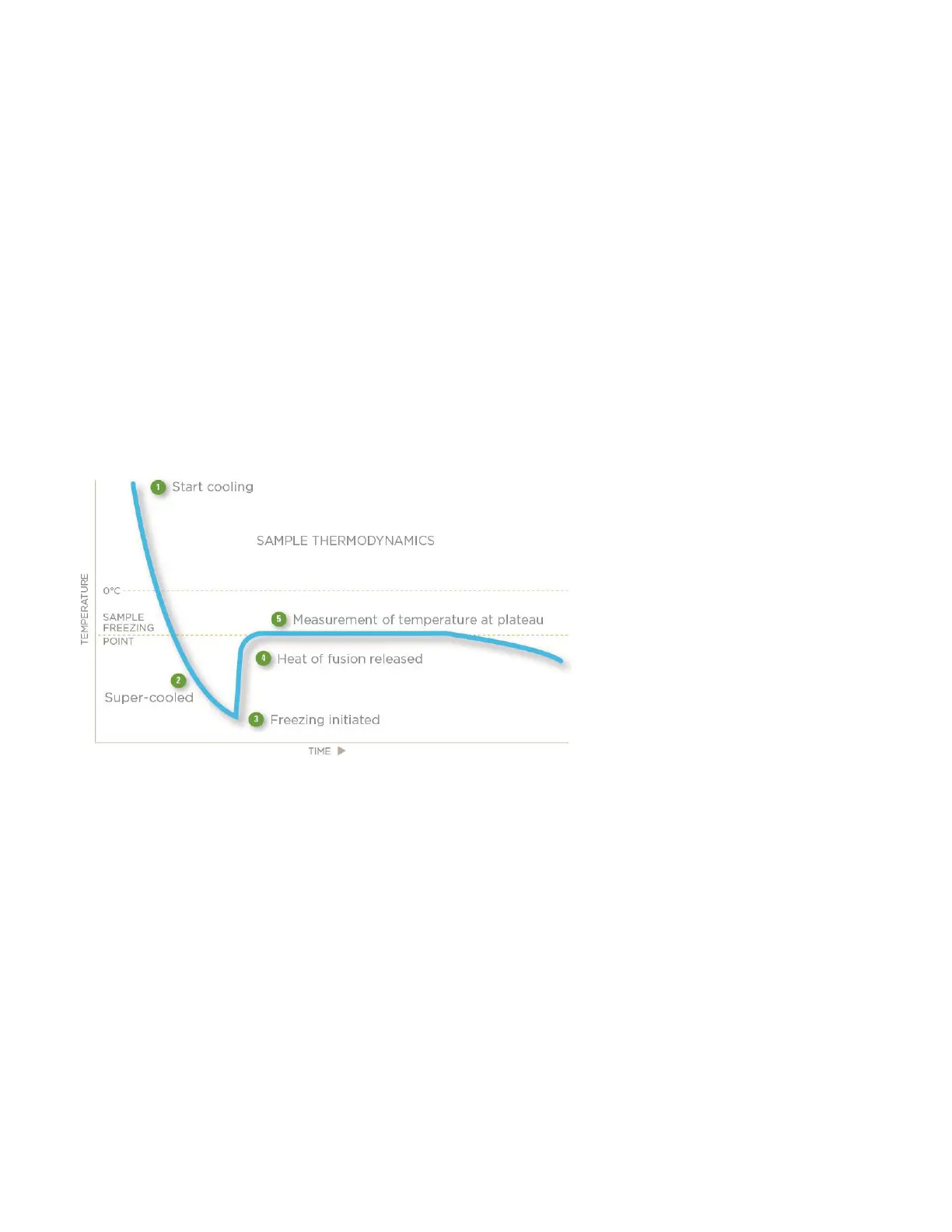
Freezing-point thermodynamics
The quickest and most precise way to measure the freezing point of a solution is to supercool it several degrees below its
freezing point and inducing crystallization of the solution via mechanical agitation. The sudden liberation of energy (heat
of fusion) causes the sample temperature to rise toward a plateau temperature, where a liquid/solid equilibrium occurs.
The equilibrium temperature is the freezing point of the solution.
The duration of the liquid/solid equilibrium phase is a function of the speed at which the heat of fusion is liberated versus
the speed at which it is dissipated to the surrounding environment. This ratio can be slowed to prolong the equilibrium
time, giving a distinct plateau measurable to 0.001 °C.
Sensitive thermistor probes monitor the sample temperature and control the thermoelectric cooling element.
Microprocessor control and automated operation minimize imprecision introduced by operator technique.
The standard freezing curve shown below illustrates the temperature of a sample as it progresses through the freezing
cycle and shows the action of the osmometer at each stage of the cycle.
 Loading...
Loading...









