vi 300-002547-00 rev7
Disposal
When an Auris product reaches the end of its useful life and your facility desires to
remove the device, contact Auris Customer Care at +1.800.434.0032 (toll-free within the
United States) or +1.650.264.6000 (Worldwide) to uninstall and appropriately dispose of
the components.
When disposing of instruments, accessories, or any of their components, follow all
applicable national and local laws and guidelines.
Regulatory Compliance with Directives and Standards
The Monarch Platform complies with the regulatory requirements of Directive 2017/745,
the Medical Device Directive of the European Economic Community (EEC).
The Monarch Platform and accessories have been tested for compliance to the following
standards:
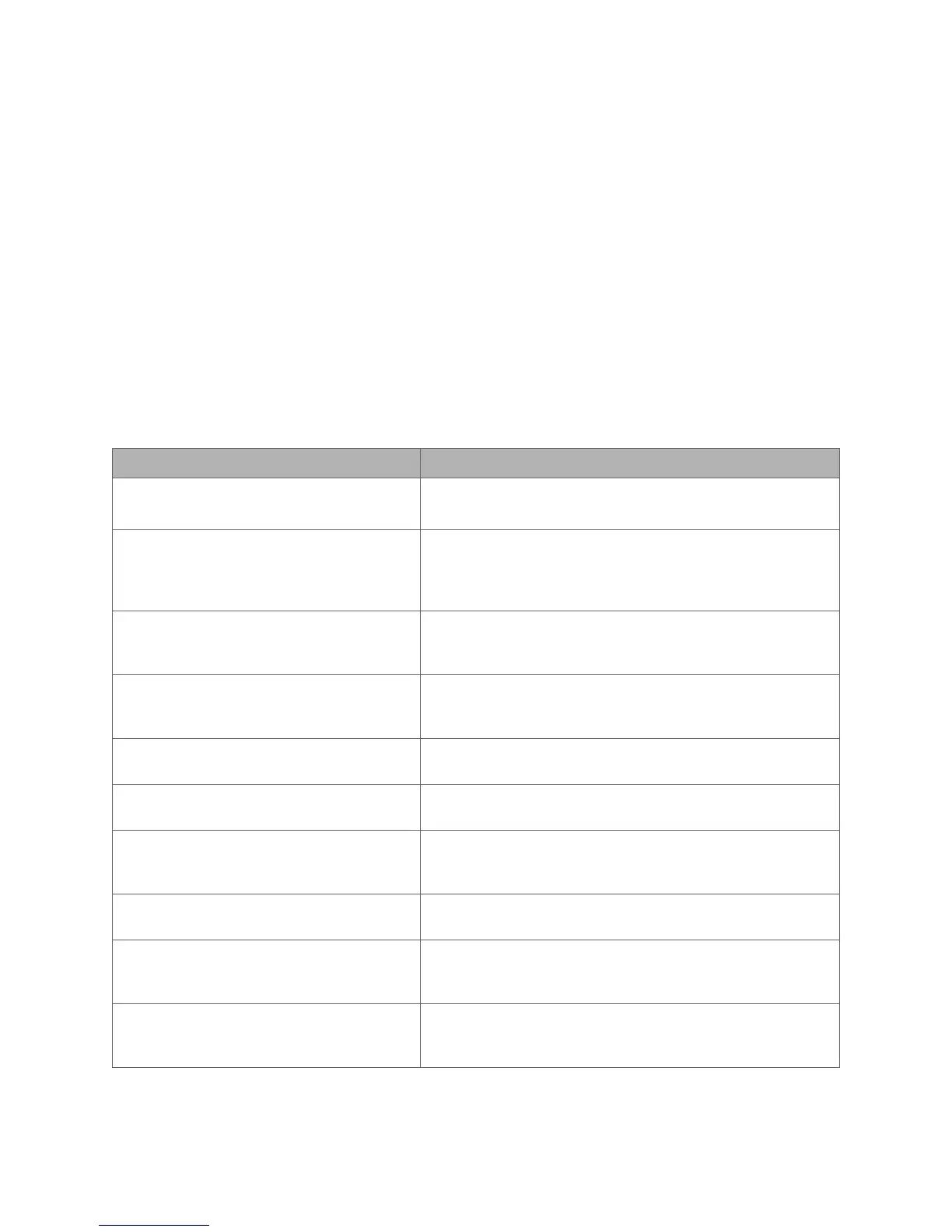
Standard Number Standard Title
AAMI/ANSI ES60601-1:2005 (Third
Edition)
Medical electrical equipment - Part 1: General
requirements for basic safety and essential performance
EN 60601-1-2:2007 (Third Edition)
Medical electrical equipment - Part 1-2: General
requirements for basic safety and essential performance –
Collateral standard: Electromagnetic compatibility –
Requirements and tests
IEC 60601-1-6:2010 (Third Edition) Medical electrical equipment – Part 1-6: General
requirements for basic safety and essential performance –
Collateral standard: Usability
IEC 60601-2-18:2009 (Third Edition)
Medical electrical equipment - Part 2-18: Particular
requirements for the basic safety and essential
performance of endoscopic equipment
IEC 62366:2007
Medical devices -- Application of usability engineering to
medical devices
IEC 62366-1:2015 Medical devices -- Part 1: Application of usability
engineering to medical devices
ISO 15223-1:2016
Medical devices -- Symbols to be used with medical device
labels, labelling and information to be supplied -- Part 1:
General requirements
ISO 14971:2007 Medical devices -- Application of risk management to
medical devices
ISO 11135:2014
Sterilization of health-care products – Ethylene Oxide –
Requirements for the development, validation, and routine
control of a sterilization process for medical devices
ISO 11607-1:2006 Packaging for terminally sterilized medical devices – Part
1: Requirements for materials, sterile barrier systems and
packaging systems
 Loading...
Loading...