Page D-2 Infinity Gamma Series VF4
Overview
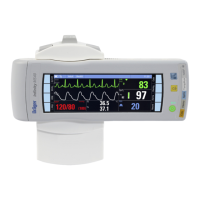
This appendix contains technical specifications for the physical
and functional aspects of the Infinity Gamma Series patient mon-
itor and its monitoring accessories. Upon request, Dräger makes
technical information required for maintenance and/or calibration
of serviceable items available to qualified technical personnel.
For specifications of wireless monitoring accessories such as
wireless LAN PC cards and access points, please refer to the doc-
umentation of the wireless component’s manufacturer, Cisco Sys-
tems, Inc.
Regulatory Compliance
Regulatory Standards Compliance
! Medical Device Directive (MDD) 93/42 EEC
! IEC 60601-1 and applicable collateral and particular stan-
dards
! EN 60601-1-2: Susceptibility -- IEC 801-2, 3, 4, and 5
! Emissions -- EN 55011, Class B
 Loading...
Loading...