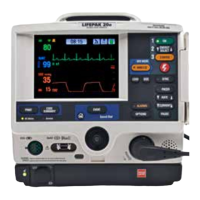
Title Service Manual, LPCR2 Page
Service Product Drawing
3323299
B
Released State Date 11/14/2017
7000545 Revision A
Sample Warranty Void Letter
The list of languages for this letter template can be found in eCentral:
3330024_A: Cust Ltr Warranty Void, LPCR2, Intl Eng
3330025_A: Cust Ltr Warranty Void, LPCR2, Swedish
3330026_A: Cust Ltr Warranty Void, CR2, Spanish
3330027_A: Cust Ltr Warranty Void, CR2, Polish
3332137_A: Cust Ltr Warranty Void, LPCR2, Norwegian
3330029_A: Cust Ltr Warranty Void, CR2, Italian
3330030_A: Cust Ltr Warranty Void, CR2, German
3330031_A: Cust Ltr Warranty Void, CR2, Finnish
3330032_A: Cust Ltr Warranty Void, CR2, Dutch
[Name]
[Business Name]
[Street Address]
[City, State, Zip]
Re: Device Type: _______
Serial Number: ______
Customer Number: ______
Dear [Name]:
On [Date], Physio-Control, Inc. has confirmed that this device was not used in accordance with
applicable operating instructions or was not used in the intended environment or setting; specifically
the device was [enter specific failure circumstance]. As such, Physio-Control is unable to certify the
long-term reliability of a device subjected to this condition. Furthermore, the warranty is now void. Therefore, it is our
recommendation that you replace this device; we cannot service the device.
We recommend that you do not use this device clinically. Physio-Control will not accept responsibility
if the device fails to function properly in the future.
Please feel free to contact [Field Service Rep; or Technical Service Rep], or me if you have any
further questions or concerns regarding this matter.
Sincerely,
PHYSIO-CONTROL, INC.
Jeffrey Laub
VP, Global Operations and Services
 Loading...
Loading...