D–2
R
R
a
a
d
d
i
i
a
a
t
t
i
i
o
o
n
n
T
T
h
h
e
e
o
o
r
r
y
y
A more detailed discussion of radiological theory can be found
in the Troxler Nuclear Gauge Safety Training Program manual,
provided at the Troxler Safety Class.
Atomic Structure
All matter is made up of atoms. For example, water has two
atoms of hydrogen (H) and one atom of oxygen (O), which in
chemical notation is written H
2
O.
An atom is made up of a dense nucleus, consisting of positively
charged protons and uncharged neutrons, surrounded by a
cloud of negatively charged electrons. Under normal
circumstances, the number of electrons in an atom equals the
number of protons. The number of protons in the atom is called
the atomic number (Z). A chemical element consists of all atoms
having the same atomic number.
The number of protons plus neutrons in the nucleus is called the
atomic mass (A). Atoms of a given chemical element can exist in
slightly different variants called isotopes that have different
atomic masses. For example, carbon-12 (C-12) is non-
radioactive and carbon-14 (C-14) is radioactive. Isotopes that
are radioactive are termed radioisotopes or radionuclides.
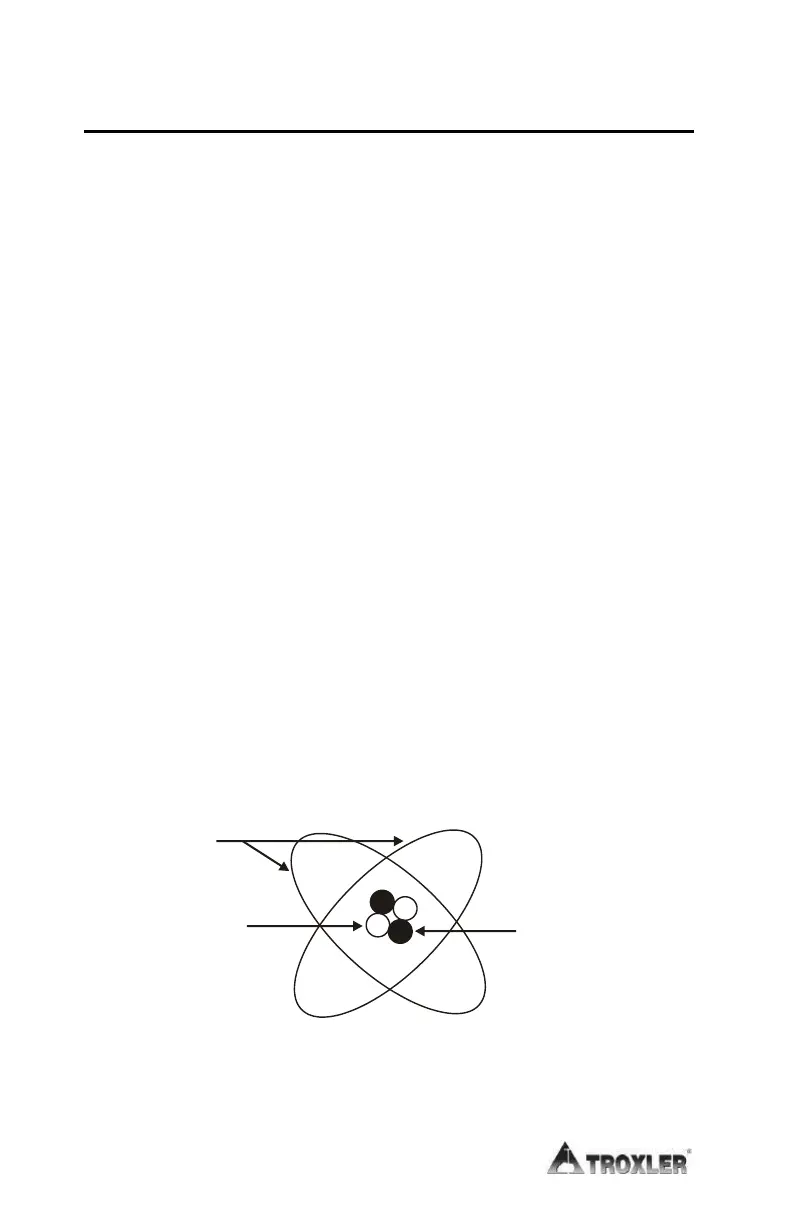
Figure 14 depicts a helium atom consisting of two protons and
two neutrons in the nucleus and two orbiting electrons.
Figure 14. Diagram of an Atom

 Loading...
Loading...











