Pannoramic SCAN II 2.2.0 User’s Guide Character Formats and Symbols
Declaration of Conformity
3DHISTECH Ltd. declares that the product Pannoramic® SCAN II slide scanner is designed and
produced with consideration of specified requirements according to the standards listed below:
IEC 61010-1:2010, “Safety Requirements for Electrical Equipment for Measurement, Control, and
Laboratory Use - Part 1: General Requirements”
IEC 61010-2-101:2015, “ Safety Requirements for Electrical Equipment for Measurement, Control,
and Laboratory Use – Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical
equipment”
IEC 61010-2-081:2015, “Safety requirements for electrical equipment for measurement, control and
laboratory use - Part 2-081: Particular requirements for automatic and semi-automatic laboratory
equipment for analysis and other purposes”
IEC 62471:2006, “Photobiological safety of lamps and lamp systems”
IEC 61326-2-6:2012, “Electrical equipment for measurement, control and laboratory use - EMC
requirements - Part 2-6: Particular requirements - In vitro diagnostic (IVD) medical equipment”
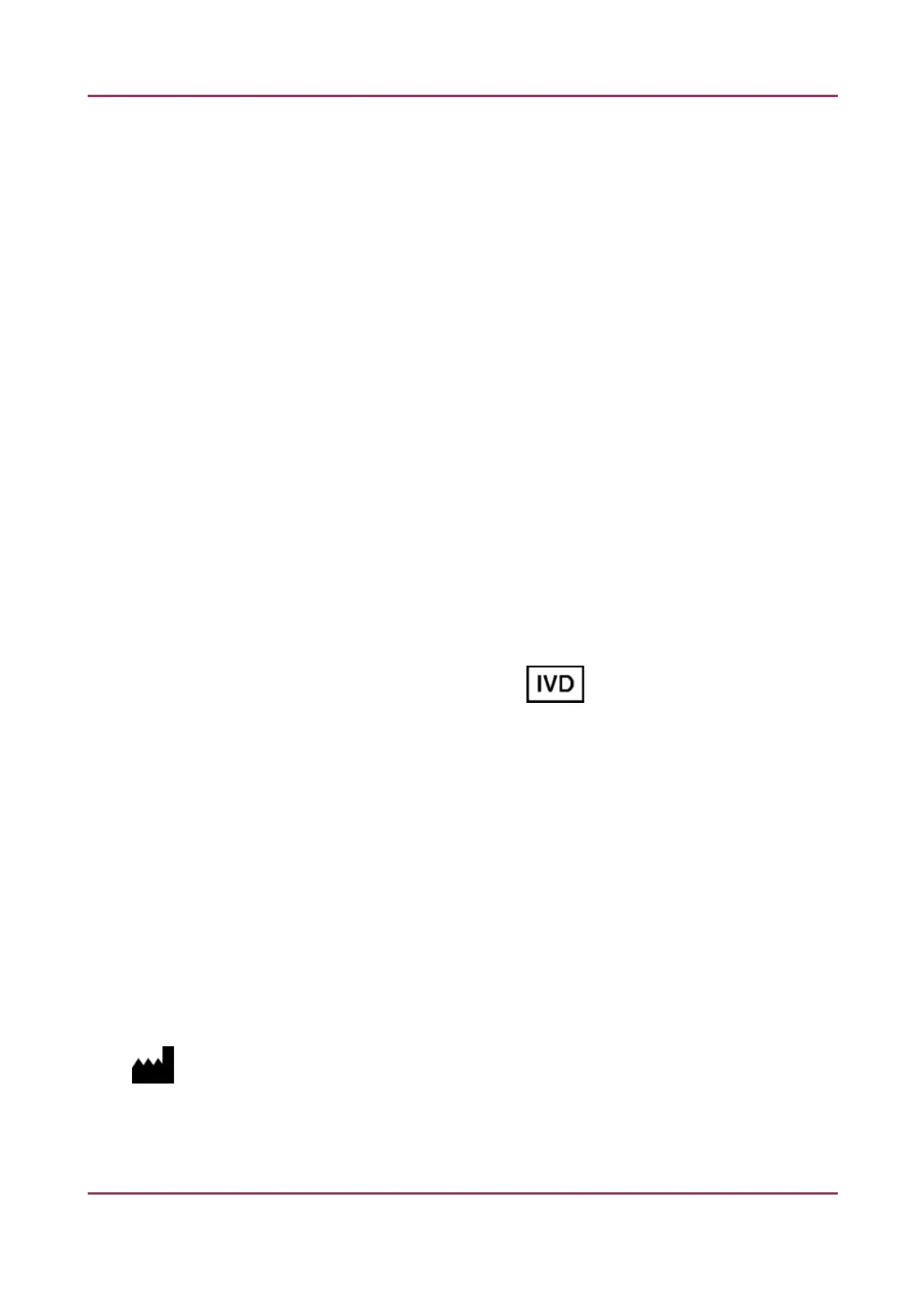
Conformity with Directive IEC 61010-2-101 is marked by on the product labeling.
There is no further operator (user) action required in case of residual risks.
Further information may be obtained from the manufacturer:
3DHISTECH Ltd.
3 Öv street
1141 Budapest – HUNGARY
January 24, 2020 - Rev. 2 3DHISTECH Ltd. 9(156)
 Loading...
Loading...