Crea - 4 Art: 714183-00T Rev. Date: 03-Aug-12
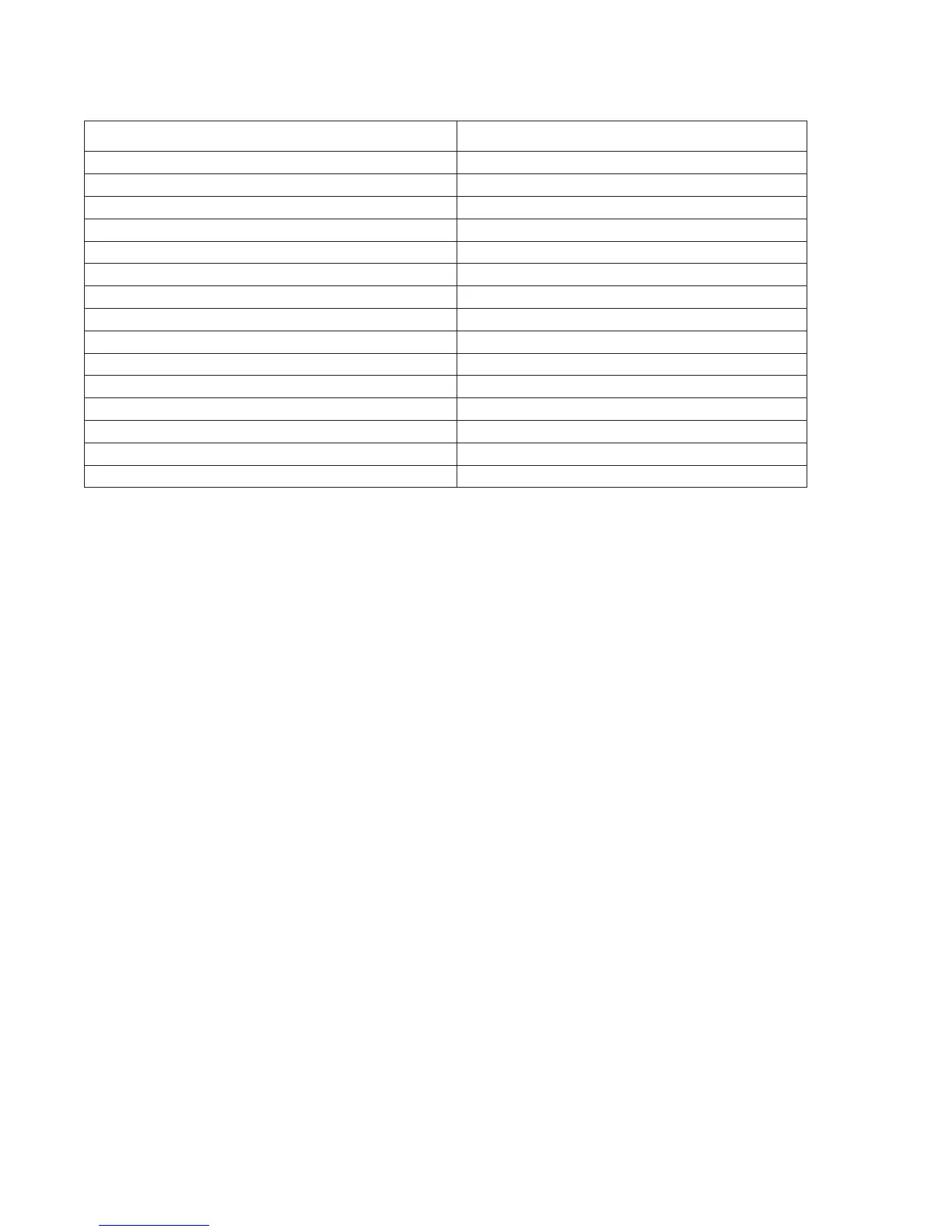
The following substances are known not to significantly interfere with the i-STAT Creatinine assay
at the stated test concentrations:
Substance Test Concentration
(mmol/L)
Acetaldehyde 0.045
7
Acetaminophen (therapeutic) 0.132
7
Acetylcysteine (therapeutic) 0.3
8,9
Bicarbonate 35.0
Bilirubin 0.342
Bromide (therapeutic) 2.5
10,11,12
Calcium Chloride 5.0
Dopamine 0.006
Formaldehyde 0.133
7
β–Hydroxybuterate 6.0
13
Lactate 6.6
Methyldopa 0.071
Pyruvate 0.31
Salicylate 4.34
Uric Acid 1.4
Notes:
1) The normal range of creatine concentration in plasma is 0.17–0.70 mg/dL (13 – 53 µmol/L) in males
and 0.35 – 0.93 mg/dL (27 – 71 µmol/L) in females
7
. Creatine may be elevated in patients using creatine
supplements, experiencing muscle trauma or other primary or secondary myopathies, taking statins for
hyperlipidemia control, or in patients with hyperthyroidism or a rare genetic defect of the creatine trans-
porter protein.
2) Hydroxyurea is a DNA synthesis inhibitor used in the treatment of various forms of cancer, sickle cell
anemia, and HIV infection. This drug is used to treat malignancies including melanoma, metastatic ovar-
ian cancer, and chronic myelogenous leukemia. It is also used in the treatment of polycythemia vera,
thrombocythemia, and psoriasis. At typical doses ranging from 500 mg to 2 g/day, concentrations of
hydroxyurea in patients’ blood may be sustained at approximately 100 to 500 µmol/L. Higher concentra-
tions may be observed soon after dosing or at higher therapeutic doses.
3) Acetaminophen has been shown to interfere at a concentration proscribed by the CLSI guideline, 1.32
mmol/L, which represents a toxic concentration. Acetaminophen at 0.132 mmol/L, which represents the
upper end of the therapeutic concentration, has been shown not to significantly interfere with i-STAT crea-
tinine results.
4) Acetylcysteine has been tested at two levels; the CLSI recommended level and a concentration of
0.30 mmol/L. The latter is 3 times the peak plasma therapeutic concentration associated with treatment
to reverse acetaminophen poisoning. APOC has not identified a therapeutic condition that would lead
to levels consistent with the CLSI recommended level. Acetylcysteine at a concentration of 10.2 mmol/L
increased i-STAT creatinine results, while acetylcysteine at a concentration of 0.3 mmol/L did not signifi-
cantly interfere with i-STAT creatinine results.
 Loading...
Loading...