PT/INR - 2 Art: 715236-00N Rev. Date: 01-Mar-12
Intended Use
The i-STAT PT, a prothrombin time test, is useful for monitoring patients receiving oral anticoagulation therapy
such as Coumadin or warfarin.
Contents
Each i-STAT PT/INR cartridge provides a sample collection chamber, sensors to detect the coagulation
endpoint and dry reagents necessary to initiate and allow coagulation. Inert matrix components and
reagents are coated on a section of the sensor channel and include the following reactive ingredients:
Reactive Ingredient Biological Source
Recombinant Tissue
Thromboplastin
Human
Heparinase I Flavobacterium heparinum
Thrombin Substrate N/A
Metrological Traceability
The i-STAT System test for Prothrombin Time (PT/INR) measures the International Normalized Ratio
(dimensionless) expressing the relative time interval required for complete activation, by thromboplastin,
of the coagulation cascade in capillary or venous whole blood for in vitro monitoring of oral anticoagulant
(Coumadin or warfarin) therapy. PT/INR values assigned to i-STAT’s controls are traceable to the World
Health Organization (WHO) international reference measurement procedures and the International Reference
Preparation recommended by the WHO.
2
i-STAT System controls are validated for use only with the i-STAT
System and assigned values may not be commutable with other methods. Further information regarding
metrological traceability is available from Abbott Point of Care Inc.
Expected Values
Test/Abbreviation Units Verified Clinical Range
Prothrombin Time/ (PT/INR) INR 0.9 - 6.0*
*The performance characteristics of the i-STAT PT/INR measurement have not been established at INRs above 6.0.
Performance Characteristics
The typical performance data summarized below were collected in healthcare facilities by healthcare
professionals trained in the use of the i-STAT System and comparative methods.
Imprecision
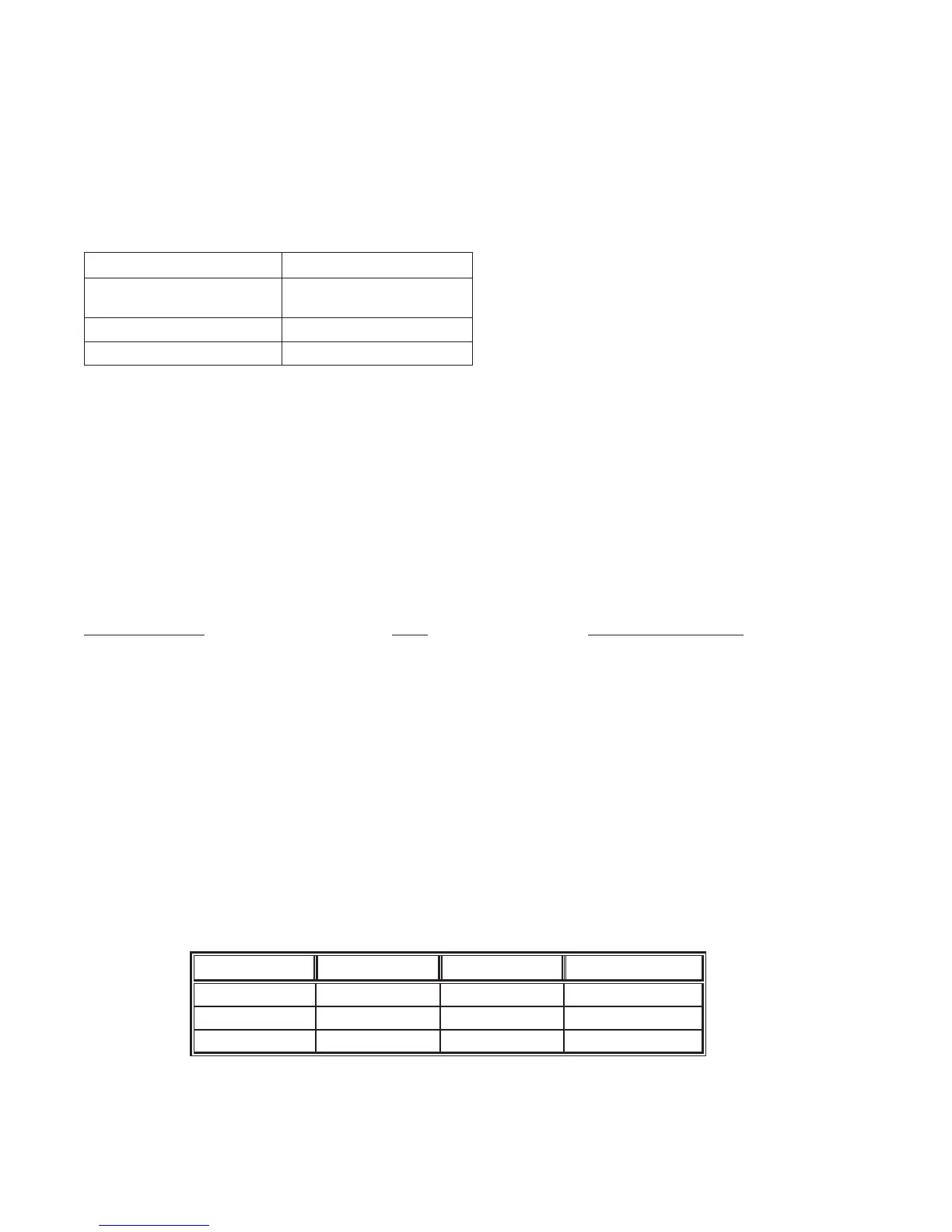
Initial studies were conducted to collect imprecision data for venous and capillary whole blood samples.
Imprecision data for venous whole blood samples were collected in duplicate at two clinical sites.
Imprecision data for capillary whole blood samples were collected in duplicate at one clinical site using a
single capillary stick. The table below summarizes this data.
Statistic Site 1 (venous) Site 2 (venous) Site 3 (capillary)
n 181 102 33
Mean (INR) 2.6 2.4 2.5
%CV 4.7% 4.0% 4.6%

 Loading...
Loading...