Rev. Date: 01-Mar-12 Art: 715236-00N PT/INR - 3
The below imprecision data for lyophilized plasma control material were collected during studies at an
Abbott Point of Care facility and during clinical trials. SD and %CV are typical of current performance.
Current Value Assignment Sheets should be referenced for applicable plasma control mean data.
Plasma Control Mean SD %CV
Level 1 1.1 (INR) 0.05 4.5%
Level 2 2.5 (INR) 0.17 6.9%
Reference Interval
In a study to determine a reference interval for PT/INR, venous samples from healthy volunteers were
collected in plastic tubes, and whole blood was analyzed with one lot of cartridges on the i-STAT System.
Capillary samples were obtained from the same volunteers using Softclick Pro (setting of 3) and analyzed
on the same cartridge lot. Reference intervals for INR in venous and capillary samples were determined
according to the CLSI Guideline C28-A2.
3
The data are summarized in the table below:
Statistic Venous whole blood Capillary whole blood
n 120 119
Mean (INR) 1.0 1.0
SD 0.1 0.1
Reference Range (INR) 0.8 - 1.2 0.8 - 1.2
Due to the many variables that may affect PT/INR results, each laboratory should establish its own
reference interval.
Method Comparison
Method comparison data were collected at the Hemostasis Reference Laboratory (Hamilton, Ontario,
Canada). Venous samples from outpatients undergoing routine oral anticoagulation therapy were collected
in plastic tubes and analyzed in duplicate on multiple lots of cartridges on the i-STAT System; plasma from
tubes containing a citrate anticoagulant were analyzed in duplicate on the comparative instruments using
Dade
®
Innovin
®
, STA Neoplastine
®
CI Plus, and the HemosIL
®
RecombiPlasTin 2G
®
reagents.
Deming regression analysis
4
was performed on the first replicate of each sample. In the method
comparison table below, n is the number of specimens in the data set, Sy.x is the standard error of the
estimate, and r is the correlation coefficient.
Method comparisons will vary from site to site due to differences in the sample handling, reagent and
instrument systems in use, and other site-specific variables. A correlation study should be performed to
establish the differences between the i-STAT PT/INR measurement and other methods used.
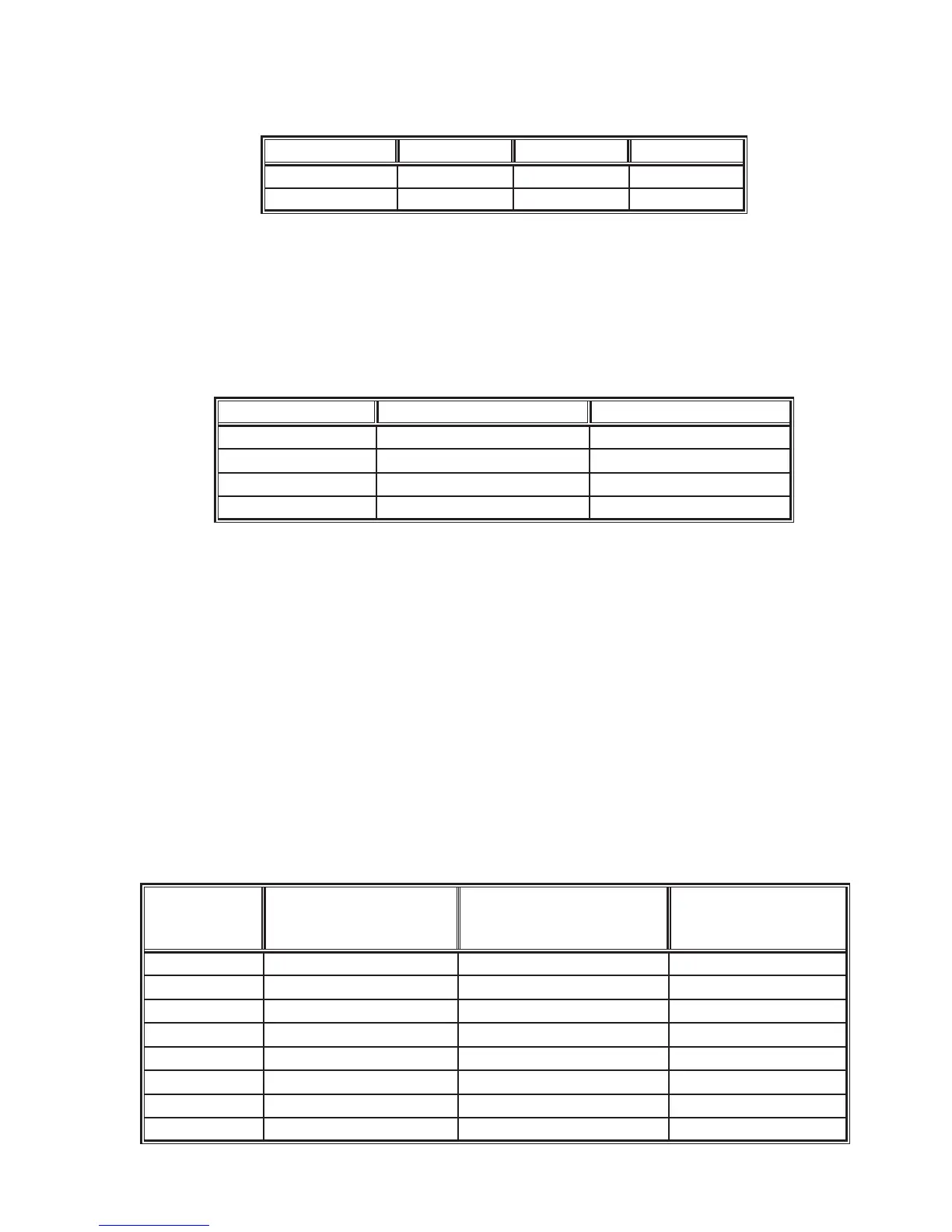
Statistic
i-STAT vs. Siemens Sysmex
®
CA-1500 and
Dade
®
Innovin
®
reagent
i-STAT vs. STA Compact
®
and
Neoplastine
®
CI Plus reagent
i-STAT vs. IL ACL 7000 and
HemosIL
®
RecombiPlasTin
2G
®
reagent
n 78 78 69
Mean (INR) 2.1 2.1 2.2
Range (INR) 0.9 - 4.5 0.9 - 4.1 0.9 – 4.0
Sx (INR) 0.843 0.772 0.840
Slope 0.981 1.074 0.972
Intercept (INR) 0.084 -0.100 0.003
r 0.963 0.964 0.962
Sy.x 0.233 0.229 0.233

 Loading...
Loading...