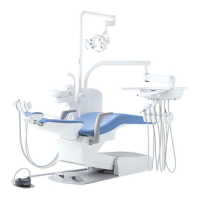
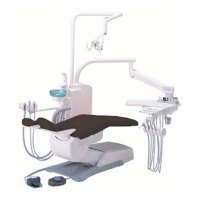
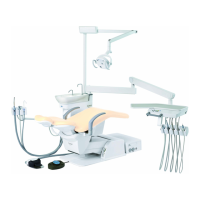
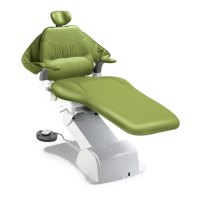
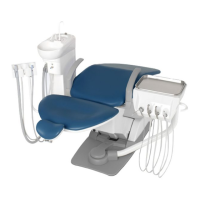
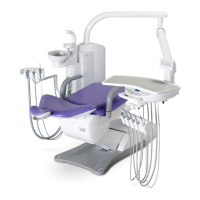
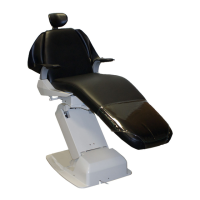
This product is an active therapeutic device intended for the exclusive use for diagnoses,
treatments and relative procedures of dentistry.
The product must be operated or handled by the qualified dentists or by dental staffs under
the supervision of the dentist.
Such dentists or dental staffs should instruct and/or assist the patients to approach to and leave
from the product.
Patients should not be allowed to operate or handle the product unless he/she is so instructed.
The product is supplied together with the handpieces like electric micromotor, air turbine
and/or motor, scaler and so on.
Compatibility of Handpieces
Use the compatible handpieces as shown on the attached list for this unit. (List of compatible
handpieces).
In case of disposal of equipment
When disposing the unit and chair, appropriately dispose complying with all current applicable
regulations and local codes.
In EU area, EU directive 2002/96/EC on waste electrical and electronic equipment (WEEE) is applied
on this product. In this directive, environment conscious recycling/abandonment is obligated.
Disposal of residue material
Please request a special contractor when you dispose amalgam.
Important Notes
In case of the troubles, please contact Takara Belmont offices or your dealers.
Do not disassemble or attempt to repair.
Disassembly, repair or modifications should only be done by a qualified repair technician.
Attempts at disassembly, repair or modifications may lead to abnormal operation and accidents.
Intended Use of the Product
Compliance with Directives
tbCOMPASS compliance with the following directives, Medical Device Directive 93/42/EEC,
RoHS Directive 2011/65/EU.
 Loading...
Loading...